- Title
-
Cortisol rapidly stimulates calcium waves in the developing trunk muscle of zebrafish
- Authors
- Das, C., Faught, E., Vijayan, M.M.
- Source
- Full text @ Mol. Cell. Endocrinol.
|
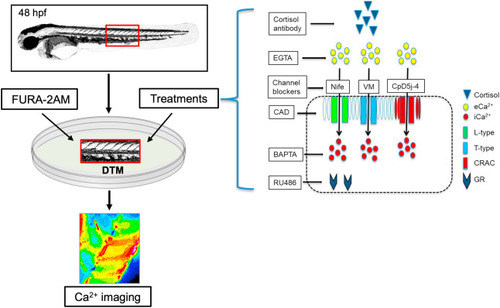
Fig. 1. Calcium imaging in zebrafish embryos. The developing trunk muscle (DTM) of 48 h post-fertilization (hpf) zebrafish embryos were used to test the rapid effects of cortisol on Ca2+ dynamics using the FURA-2AM method. The treatments included cortisol antibody to sequester cortisol, EGTA to chelate extracellular Ca2+ (eCa2+), BAPTA-AM (BAPTA) to chelate intracellular Ca2+ (iCa2+), and RU486 as a GR antagonist. Also, Ca2+ channel blockers, including nifedipine (Nife) to block L-type channel, verapamil (VM) to block T-type channel, cadmium as a non-specific blocker of plasma membrane Ca2+ channels, and CpD5J-4 to block the CRAC channels were utilized. See materials and methods for details. |
|
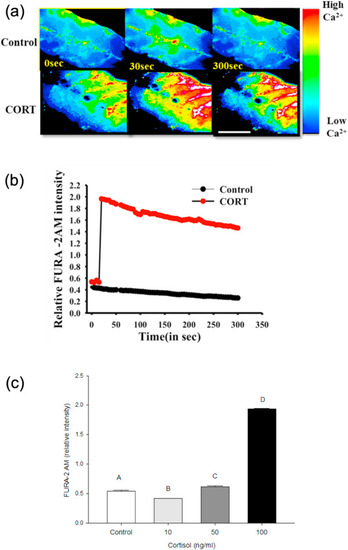
Fig. 2. Cortisol rapidly stimulates [(Ca2+)i] wave in zebrafish muscle. (a) Representative images of the developing trunk muscle (DTM) loaded with the ratiometric dye FURA-2AM either with (100 ng/ml) or without the addition of cortisol. (b) A representative line graph of the temporal calcium dynamics in response to cortisol (CORT) addition over a 5 min period. FURA-2AM intensity was recorded at 5 s intervals. (c) Graph showing the ratiometric FURA-2AM intensity with different concentrations of cortisol (10, 50 and 100 ng/ml) at 30 s post-cortisol addition. All bars represent mean ± SEM (n = 4–5 independent embryos); bars with different letters are significantly different; one-way ANOVA; Holm Sidak post hoc test; p < 0.05. |
|
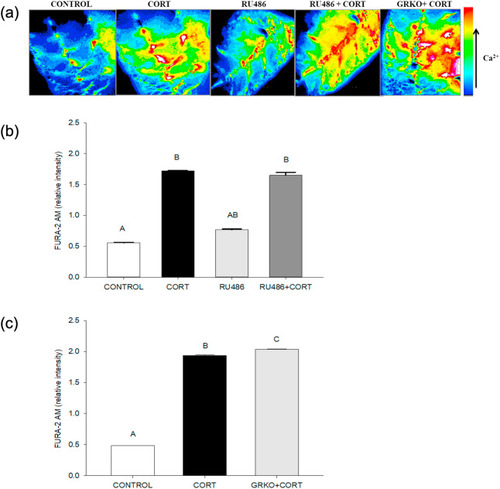
Fig. 3. The cortisol-mediated [(Ca2+)i] increase is independent of glucocorticoid receptor. (a) Representative images of the developing trunk muscle (DTM) loaded with the ratiometric dye FURA-2AM and treated with either vehicle (CONTROL), cortisol (CORT), RU486 or a combination of cortisol and RU486 (RU486+CORT), as well as cortisol treatment of the DTM from GRKO zebrafish (GRKO + CORT). (b) Graph showing the ratiometric FURA-2AM intensity in the DTM treated with either cortisol, RU486 or a combination of cortisol and RU486 at 30 s post-cortisol exposure. (c) Graph showing the ratiometric FURA-2AM intensity in wildtype or GR knockout (GRKO) DTM at 30 s post-cortisol addition. All bars represent mean ± SEM (n = 4–5 independent embryos); bars with different letters are significantly different; one-way ANOVA; Holm Sidak post hoc test; p < 0.05. |
|
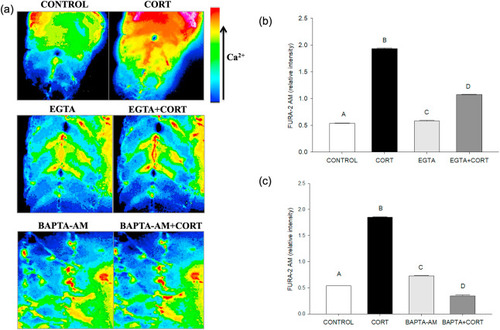
Fig. 4. Cortisol-mediated [(Ca2+)i] rise involves both intracellular and extracellular Ca2+. (a) Representative images of the developing trunk muscle (DTM) loaded with the ratiometric dye FURA-2AM and treated with either vehicle (CONTROL), cortisol (CORT), EGTA, BAPTA-AM or a combination of cortisol with EGTA (EGTA + CORT) or BAPTA-AM (BAPTA-AM + CORT). (b) A bar graph showing the ratiometric intensity at 30 s with extracellular calcium chelator EGTA either with or without cortisol. (c) A bar graph showing the ratiometric intensity at 30 s with an intracellular chelator BAPTA-AM either with or without cortisol. All bars represent mean ± SEM (n = 4–5 independent embryos); bars with different letters are significantly different; one way ANOVA; Holm Sidak post hoc test; p < 0.05. Abbreviations: Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′- tetraacetic acid (EGTA), 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxy methyl ester (BAPTA-AM). |
|
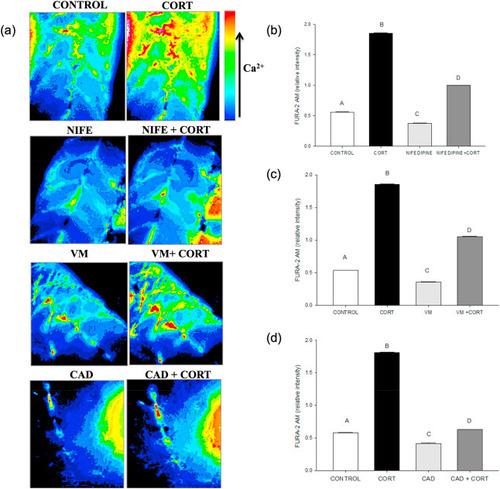
Fig. 5. Cortisol-mediated [(Ca2+)i] rise involves plasma membrane Ca2+ channels. (a) Representative images of the developing trunk muscle (DTM) loaded with the ratiometric dye FURA-2AM and treated with either vehicle (CONTROL), cortisol (CORT) or the Ca2+ channel blockers nifedipine (NIFE), verapamil (VM) or cadmium (CAD) either without or with cortisol (NIFE + CORT; VM + CORT; CAD + CORT). (b) A bar graph showing the ratiometric intensity at 30 s with the L-type calcium channel inhibitor, nifedipine either with or without cortisol. (c) A bar graph showing the ratiometric intensity at 30 s with the T-type calcium inhibitor verapamil either with or without cortisol. (d) A bar graph showing the ratiometric intensity at 30 s with non-specific calcium channel inhibitor cadmium either with or without cortisol. All bars represent mean ± SEM (n = 4–5 independent embryos); Bars with different letters are significantly different; one way ANOVA; Holm Sidak post hoc test; p < 0.05. |
|
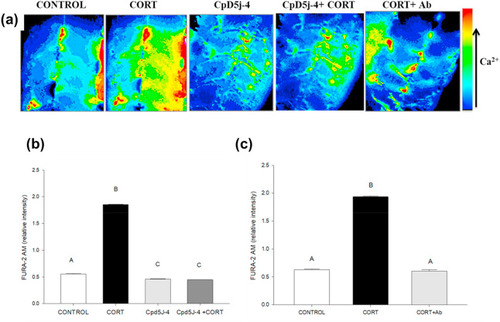
Fig. 6. Cortisol-mediated [(Ca2+)i] rise involves calcium release-activated calcium channel (CRACC) activation. (a) Representative images of the developing trunk muscle (DTM) loaded with the ratiometric dye FURA-2AM and treated with either vehicle (CONTROL), cortisol (CORT) without or with antibody for quenching (CORT + Ab), and CRACC inhibitor (CpD5J-4) without or with cortisol (CpD5J-4+CORT). (b) A bar graph showing the ratiometric intensity at 30 s with the CRACC inhibitor CpD5J-4 either with or without cortisol. (c) A bar graph showing the ratiometric intensity at 30 s with CORT either without or with the antibody (CORT + Ab). All bars represent mean ± SEM (n = 4–5 independent embryos); bars with different letters are significantly different; one way ANOVA; Holm Sidak post hoc test; p < 0.05. |
|
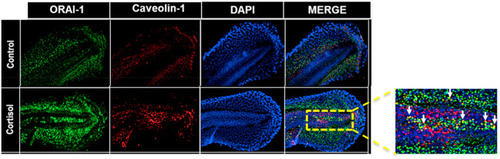
Fig. 7. Cortisol rapidly affects Orai1 expression in the developing trunk muscle. (a) Spatial changes in Orai1 (ORAI-1), the pore forming subunit of CRACC, expression in zebrafish embryo. ORAI-1 (green), Caveolin-1(red) and DAPI (blue). ORAI-1 expression in the developing trunk muscle (DTM) is enhanced in the cortisol exposed muscles, and the colocalization between ORAI-1 and caveolin-1 is shown as an inset (yellow), and the white arrows indicate colocalization. |
|
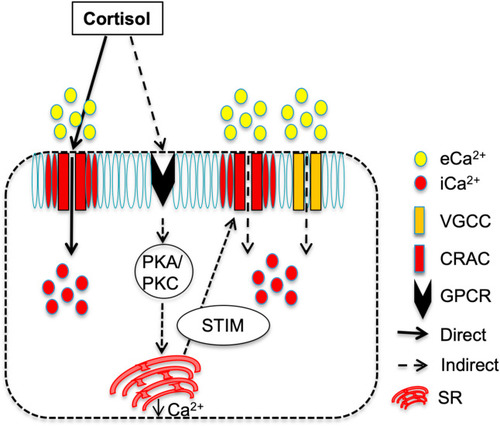
Fig. 8. Proposed model for the rapid cortisol action on [(Ca2+)i] in the developing trunk muscle (DTM) of zebrafish. We propose that cortisol-mediated rapid rise in [(Ca2+)i] occur via two pathways: i) A direct effect of cortisol by binding to the CRAC channel and modulating the pore (Orai-1) opening to allow extracellular Ca2+ (eCa2+) entry causing the rapid intracellular Ca2+ (iCa2+) rise, and ii) an indirect effect of cortisol that may include the activation of a yet unknown membrane GPCR resulting in either the gating of VGCC and/or the activation of PKA/PKC signaling leading to the release of Ca2+ from the sarcoplasmic reticulum (SR). The drop in SR Ca2+ stores activates the Ca2+ sensor STIM, which interacts with the CRAC channel to cause eCa2+ entry leading to a rise in [(Ca2+)i] in the DTM. Abbreviations: [(Ca2+)i] – cytosolic free calcium levels, CRAC- calcium release-activated Ca2+ channel, VGCC - voltage-gated Ca2+ channels, STIM-stromal interaction molecule, PKA-protein kinase A, PKC – protein kinase C, GPCR-G protein-coupled receptor. |
Reprinted from Molecular and Cellular Endocrinology, 520, Das, C., Faught, E., Vijayan, M.M., Cortisol rapidly stimulates calcium waves in the developing trunk muscle of zebrafish, 111067, Copyright (2020) with permission from Elsevier. Full text @ Mol. Cell. Endocrinol.