- Title
-
Spatio-temporal expression and distribution of collagen VI during zebrafish development
- Authors
- Tonelotto, V., Trapani, V., Bretaud, S., Heumüller, S.E., Wagener, R., Ruggiero, F., Bonaldo, P.
- Source
- Full text @ Sci. Rep.
|
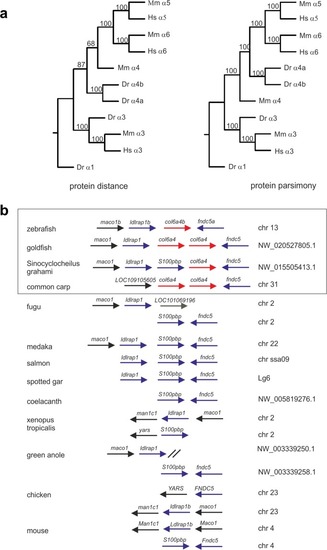
Phylogenetic analysis of zebrafish ColVI genes. ( |
|
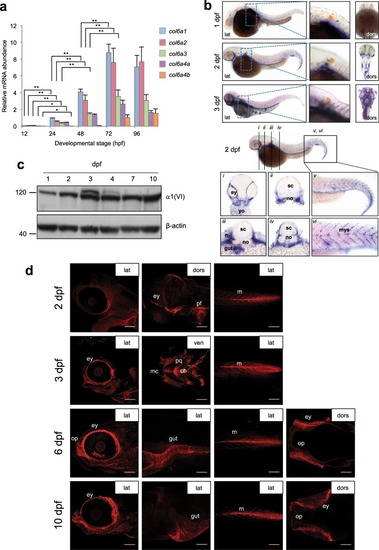
ColVI expression in zebrafish at different developmental stages. ( |
|
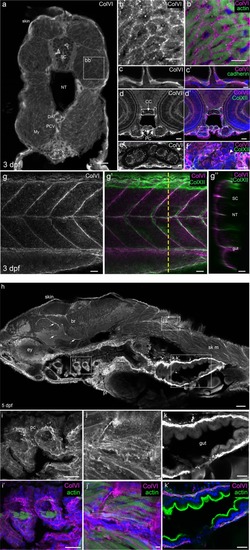
Characterization of ColVI deposition in 3- and 5-dpf developing zebrafish. ( EXPRESSION / LABELING:
|
|
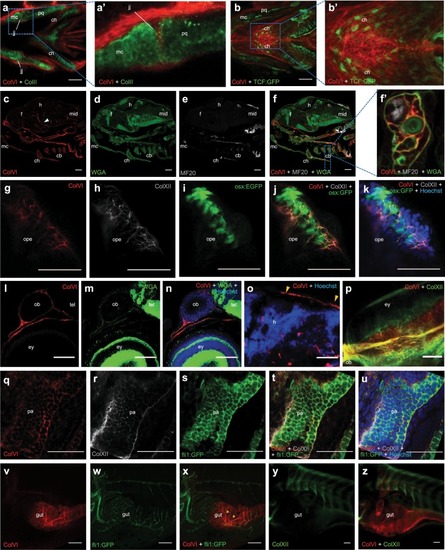
Spatio-temporal pattern of ColVI distribution in larvae from wild-type animals and transgenic reporter fish lines. ( EXPRESSION / LABELING:
|
|
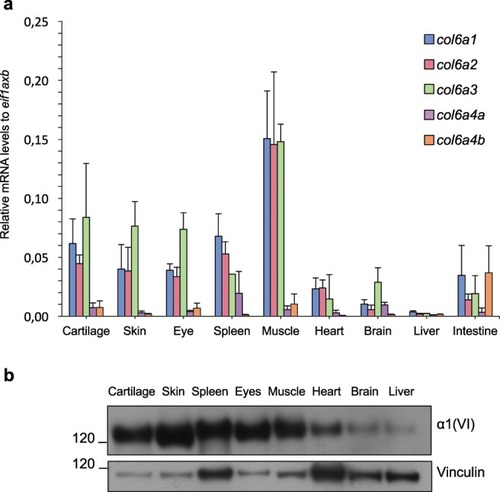
ColVI expression in adult zebrafish tissues. ( |
|
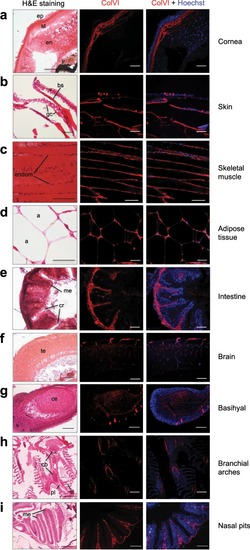
Characterization of ColVI deposition in adult zebrafish. ( EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|