- Title
-
Periderm invasion contributes to epithelial formation in the teleost pharynx
- Authors
- Teixeira Rosa, J., Oralová, V., Larionova, D., Eisenhoffer, G.T., Eckhard Witten, P., Huysseune, A.
- Source
- Full text @ Sci. Rep.
|
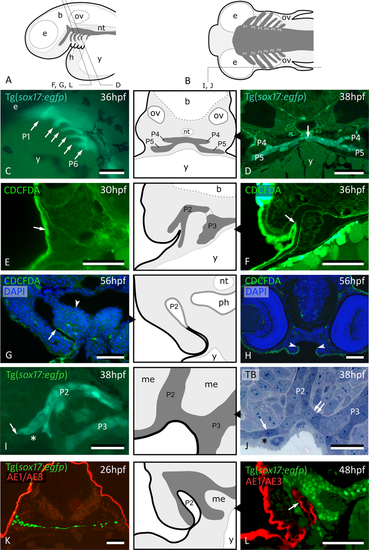
Peridermal cells invade pouch 2. (A,B) Generalized scheme of zebrafish pouches (dark grey) in a lateral (A) and horizontal (B) view, with sections D-L as indicated. (C) Lateral view of Tg(sox17:egfp) embryo showing pouches 1 to 6 (compare to (A)). (D) GFP labeled endodermal cells along the midline (white arrow) extend into pouches 4 and 5 (P4 and P5, resp.); corresponding schematic attached. (E) CDCFDA labeling at 26 hpf, sacrifice at 30 hpf; labeled cells are exclusively located in the superficial skin layer or periderm (arrow, level of pouch 2). (F) CDCFDA labeling at 26 hpf, sacrifice at 36 hpf; peridermal cells have partially invaded pouch 2 (arrow); corresponding schematic attached. (G) CDCFDA labeling at 26 hpf, sacrifice at 56 hpf; peridermal cells (arrow) line the now open gill slit; elsewhere the pharyngeal epithelium remains free of peridermal cells (arrowhead); corresponding schematic attached. (H) CDCFDA labeled cells only cover part of the epithelial lining of the mouth. Their boundary is indicated by arrowheads and coincides with the reduction of two to one epithelial cell layer. (I,J) Contact area of pouches 2 and 3 with skin; note that sox17+ cells spread out (arrows) and appear to displace the basal cell layer. In this way, the distal parts of the pouches even become connected (double arrow). Only periderm (asterisks) covers the expansion of sox17+ endodermal cells. Corresponding schematic attached. (K,L) Immunolabeling using the pan-cytokeratin marker AE1/AE3 on Tg(sox17:egfp) embryos labels periderm only at 26 hpf (K), but also marks the lining of P2 at 48 hpf (arrow) (L); corresponding schematic attached. In all schemes, endoderm is dark gey, periderm is a black line. (C) Whole mount embryo; (D–H,K,L) cross sections; (I,J) sagittal sections. b: brain; e: eye; h: heart; me: mesenchyme; nt: notochord; ov: otic vesicle; P2 – P6: pouches 2 to 6; ph: pharyngeal lumen; TB: toluidine blue staining; y: yolk. Scale bar (C) = 500 μm, (D–H,K,L) = 50 μm, (I,J) = 20 µm.
|
|
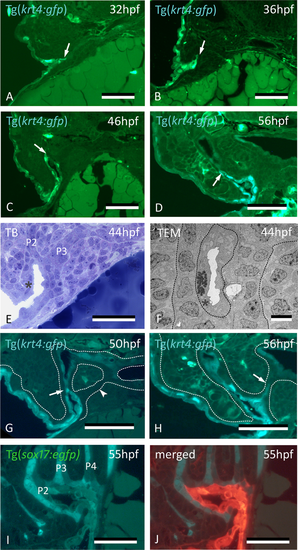
Peridermal cells sequentially invade pouches 2 to 6 but do not reach the midline endoderm. (A–C) krt4+ cells are present in progressively deeper parts of pouch 2 (P2) (arrows). (D) Peridermal cells are arrested approximately halfway the pouch (arrow). (E,F) At 44 hpf P2 is open to the exterior. Peridermal cells extend inside the pouch (black stars); a lumen forms only where these cells cover the endodermal layer. In (F), outer dotted lines mark contour of the pouch; inner dotted line marks contour of peridermal cells. (G) While peridermal cells have entered through P2 (arrow), krt4+ cells remain excluded from P3 (arrowhead) and more posterior pouches. (H) At 56 hpf, peridermal cells have started to enter P3 (arrow), and progressively extend inwards. (I,J) Double transgenics Tg(sox17:egfp;krt4:tomato) show opening of P2 with periderm entering and covering sox17+ endodermal cells (I, green channel only; J, overlay). P2–P6: pouches 2 to 6. (A–H) cross sections, (I,J) sagittal sections. TB: toluidine blue staining. Scale bars (A–E) and (G–J) = 50 μm, (F) = 5 μm.
|
|
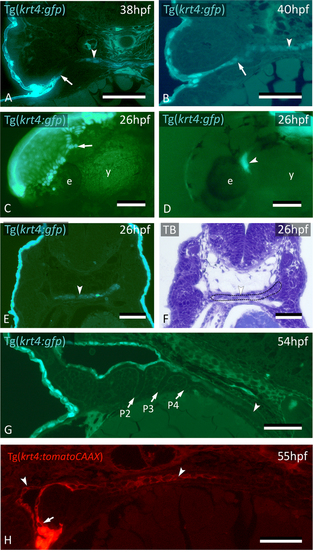
krt4+ cells expand along the midline and connect to invaded peridermal cells. (A) At 38 hpf, krt4+ cells are visible along the midline (arrowhead), separated from invading peridermal cells (arrow). (B) Shortly after, the midline krt4+ cells (arrowhead) make contact with peridermal cells that have entered through P2 (arrow). (C) Lateral view on Tg(krt4:gfp) embryo in the early phase of periderm removal with EDTA. Part of the periderm is still attached (arrow). (D) At an advanced stage of EDTA treatment, the periderm is completely removed, and an internal krt4+ structure has become visible (arrowhead). (E,F) This internal krt4+ structure (arrowhead on E) coincides with the midline endoderm at the level of pouch 1–2 (arrowhead on F). (G) By 54 hpf, midline krt4+ cells cover the entire pharynx lining (boundary indicated by arrowhead). P2 is wide open (at another level of sectioning) but other pouches (P3–P6) are still free of krt4+ cells. (H) Double transgenic embryo Tg(sox17:egfp;krt4:tomato) of 55 hpf shows lining of pharyngeal epithelium with krt4+ cells (arrowheads) (red channel only). Note continuity, albeit with sharp boundary (arrow), with peridermal cells entering via P2. (A,B,E,F) Cross sections; (C,D) whole mount embryos; (G,H) sagittal section. TB: toluidine blue staining. e: eye; y: yolk; P2–P6: pouches 2 to 6. Scale bars (A,B,E–G) = 50 μm; (C,D) = 100 μm; (H) = 20 µm.
|
|
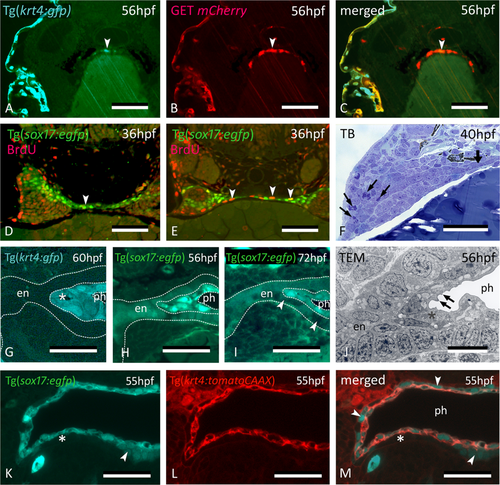
Midline krt4+ cells are distinct from peridermal and endodermal cells. (A–C) In embryos of GET-periderm x Tg(krt4:gfp) line, at 56 hpf, both the periderm and the midline krt4+ cells (arrowhead) strongly express mCherry in patterns overlapping with the krt4:GFP + cells. (D,E) Mitoses (arrowheads) revealed by BrdU pulse labeling at 36 hpf in the midline endoderm at more anterior (D) and posterior (E) cross sectional level. (F) Abundant mitoses in the distal part of the pouch (arrows) but not in the midline endoderm (thick arrow). (G) Midline cells (star) maintain a strong krt4+ expression at 60 hpf. (H,I) Downregulation of sox17+ expression in the endodermal layer (delimited by the outer dotted line in H, and by arrowheads in I) but upregulation in the superficial layer surrounding the pharyngeal lumen (delimited by inner two dotted lines). (J) Cells of the superficial layer are flattened and more electron-dense in TEM than the basal endodermal layer, composed of cuboidal cells, and they develop microridges (arrows). Epidermis to the left; midline to the right. (K–M) Double transgenic embryo Tg(sox17:egfp;krt4:tomato) of 55 hpf; krt4+ cells are positive for sox17; conversely, only some cells of the basal endodermal layer have retained a sox17 signal (arrowheads). (A–M) cross sections. en: endodermal layer; ph: pharyngeal lumen; TB: toluidine blue staining. Scale bars (A–F) = 50 μm, (G–I) = 25 µm, (J–M) = 10 µm.
|
|
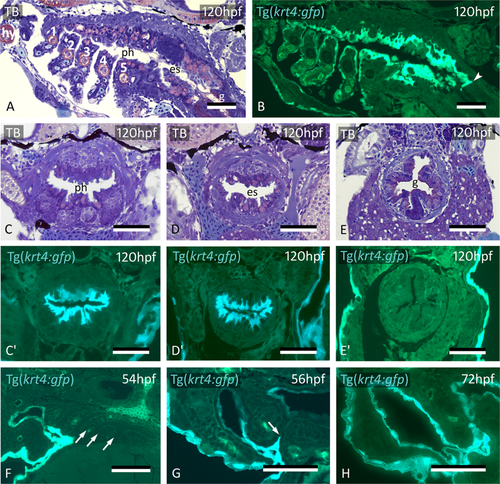
Midline krt4+ cells cover pharynx and esophagus, and meet peridermal cells invading through P3-P6. (A,B) krt4+ cells cover hyoid arch (hy) and branchial arches 1 to 5 (numbered 1–5), as well as roof and floor of the pharynx and esophagus. Note sharp boundary (arrowhead) between krt4+ esophagus and krt4- lining of the gut. (C,C’,D,D’) krt4+ cells cover the lining of the pharynx (C,C’) and the esophagus (D,D’), resp. Note long cell extensions issuing from the krt4+ cells (E,E’) Absence of krt4+ cells in the gut coincides with single-layered epithelium. (F) At 54 hpf, pouches 3–6 are still free of krt4+ cells. (G,H) Progressive invasion of P3 (arrow) by peridermal cells that meet midline krt4+ cells. (A,B,F) sagittal sections, (C–E’,G,H) cross sections. es: esophagus; g: gut; ph: pharyngeal lumen; TB: toluidine blue staining. Scale bars (A–H) = 50 μm.
|
|
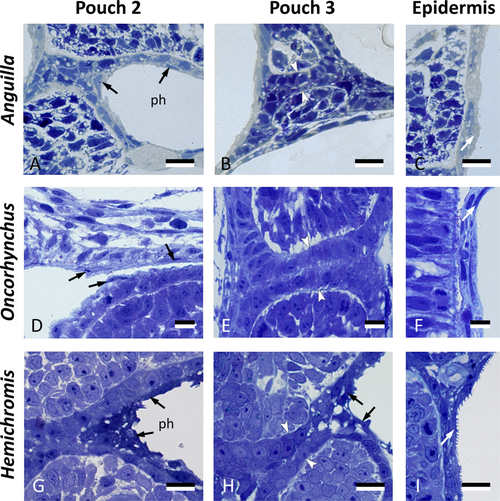
Cells resembling periderm cells in the pharynx of other teleost species. Cross sections through the region of pouch 2, pouch 3 and comparison to the skin in three teleost species: Anguilla anguilla (total length, TL, 6.0 mm), Oncorhynchus tshawytscha (unhatched embryo, 15 dpf), and Hemichromis bimaculatus (TL 4.0 mm, 1 day post-hatching, dph). Pouch 2 (A,D,G) is lined by flattened cells (black arrows) overlying a layer of cuboidal cells. Where pouch 3 is not open yet (B,E), only a double layer of cuboidal endodermal cells is present (between white arrowheads). Where pouch 3 starts to open (H), similar cells can be seen to cover the pouch endoderm (black arrows). Note resemblance, for each of the three species, to peridermal cells in the superficial skin cover (white arrows, C,F,I). In (A,B,D,E,G,H), the epidermis is to the left and the pharyngeal lumen to the right; in (C,F,I) the external surface is to the right. (A–I) cross sections. ph: pharyngeal lumen. Scale bars (A–I) = 10 μm.
|