- Title
-
Excessive feedback of Cyp26a1 promotes cell non-autonomous loss of retinoic acid signaling
- Authors
- Rydeen, A., Voisin, N., D'Aniello, E., Ravisankar, P., Devignes, C.S., Waxman, J.S.
- Source
- Full text @ Dev. Biol.
|
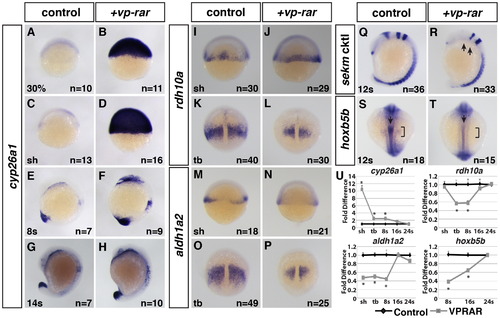
VP-RAR promotes a prolonged increase in cyp26a1 expression. (A–H) Wholemount ISH shows a dramatic increase in cyp26a1 expression in vp-rar mRNA injected embryos compared to WT siblings at 30% epiboly through 14 s stage. (I–P) ISH of RA synthesizing genes rdh10a and aldh1a2 shows modestly decreased expression in vp-rar mRNA injected embryos compared to WT at the shield stage (I,J,M,N), with dramatic decreases found by the tailbud stage (K,L,O,P). (Q,R) ISH using a six6b (anterior diencephalon), eng2a (midbrain–hindbrain boundary), egr2b (rhombomeres 3 and 5), and myod1 (adaxial cells and somites) cocktail (referred to here at sekm cktl) shows a lack of eng2a and severely reduced six6b and rhombomere 3 (anterior egr2b) in vp-rar mRNA injected compared to WT. Arrows in R indicate the loss of eng2a and reduced rhombomere 3. (S,T) Expression of hoxb5b is decreased in the lateral plate mesoderm (brackets) and anterior spinal cord (arrows) in vp-rar mRNA injected embryos compared to WT siblings. (U) RT-qPCR with cyp26a1, rdh10a, aldh1a2, and hoxb5b expression in vp-rar mRNA injected embryos at the shield (sh) through 24 s stages. Asterisks indicate statistically different from control (p<0.05) using Student′s t-test. |
|
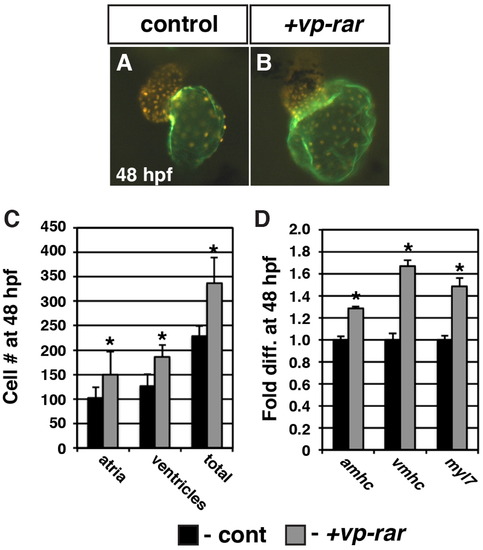
VP-RAR expression promotes increased cardiomyocyte number. (A, B) The hearts from vp-rar mRNA injected Tg(myl7:DsRed-NLS) embryos are enlarged relative to siblings. Images are frontal views. Red alone indicates ventricular cells. Atrium is indicated in green (S46 antibody). (C) Cell counts of vp-rar mRNA injected embryos at 48 hpf reveal an increase in both atrial and ventricular cardiomyocytes relative to control sibling embryos. Control n=10. Vp-rar injected n=7. (D) RT-qPCR at 48 hpf of cardiac markers amhc, vmhc, and myl7 shows increased expression in vp-rar mRNA injected embryos compared to control siblings. Asterisks indicate statistically different from control (p<0.05) using Student′s t-test. |
|
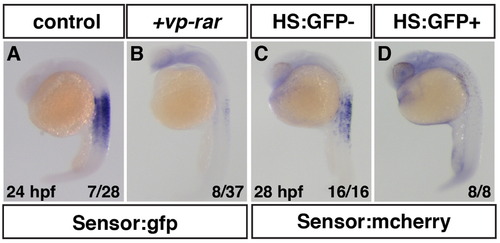
Embryonic RA is decreased in VP-RAR expressing embryos. (A, B) vp-rar mRNA injected into Tg(β-actin:GDBD-RLBD);(UAS:EGFP) embryos shows significant loss of GFP expression indicating embryonic RA is reduced compared to WT. 25% (n=7 of 28) of control embryos from the Tg(β-actin:GDBD-RLBD);(UAS:EGFP) outcrossed to a WT adult showed strong RA sensor expression, while 22% (n=8 of 37) of vp-rar mRNA injected embryos had dramatically reduced RA sensor expression. (C,D) RA levels are greatly diminished after heat-shock induction of the egfp-vp-rar transgene in Tg(β-actin:GDBD-RLBD);(UAS:mCherry) embryos compared to siblings without the heat-shock inducible transgene. |
|
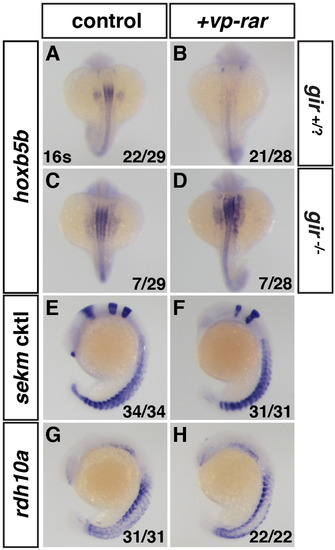
Cyp26a1 is required for induction of RA loss of function. (A,C) Gir-/- embryos have expanded hoxb5b expression relative to their siblings. (B,D) Gir-/- embryos injected with vp-rar mRNA have expanded hoxb5b expression, while the remaining gir+/? Embryos lose hoxb5b expression. (E–H) ISH for the sekm cktl and rdh10a in embryos from a gir+/- adult cross show that vp-rar mRNA injected embryos still showed brain defects and rdh10a expression was unchanged. |
|
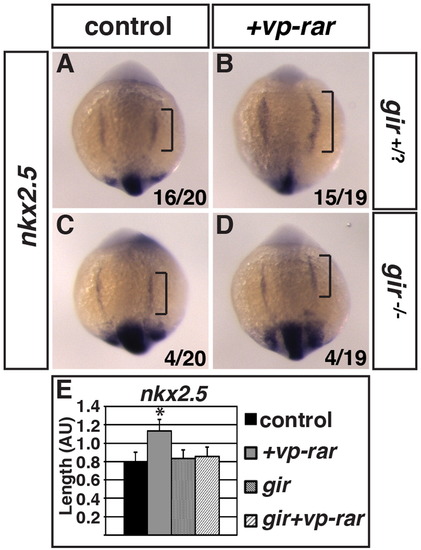
Cyp26a1 is required for increased cardiac specification in VP-RAR expressing embryos. (A–D) ISH of nkx2.5 in embryos obtained from a gir+/- adult cross with and without vp-rar mRNA injection. nkx2.5 expression is increased in vp-rar mRNA injected gir+/? embryos compared to their uninjected siblings, while vp-rar mRNA injected gir-/- embryos show no difference compared to uninjected gir-/- siblings. (E) Quantification of nkx2.5 expression length. Asterisks indicate statistically different from control (p<0.05) using Student′s t-test. |
|
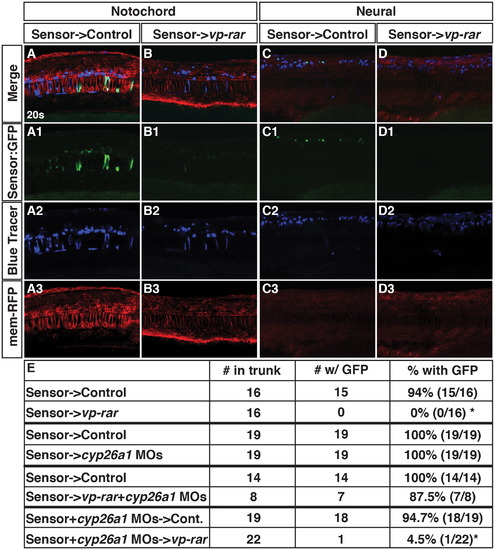
Cyp26a1 has cell non-autonomous consequences on RA signaling levels within the local environment. (A–A3, C–C3) Tg(β-actin:GDBD-RLBD);(UAS:EGFP) donor cells transplanted into WT hosts injected with mrfp mRNA have EGFP expression in the notochord and spinal cord. (B–B3, D–D3) Tg(β-actin:GDBD-RLBD);(UAS:EGFP) donor cells transplanted vp-rar mRNA injected hosts with mrfp mRNA have severely dampened GFP expression. (E) Table showing summary of blastula transplantation results. Asterisks indicate statistically different from controls (p<0.05) using a modified chi-square test. |
|
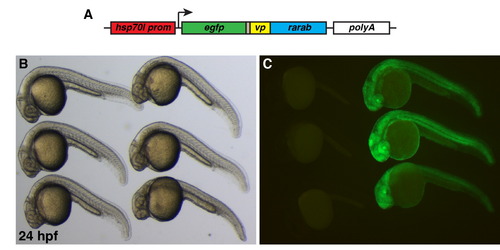
Characterization of Tg(hsp70l:EGFP-VP-RAR)ci104 line (A) Schematic of hsp70l:EGFP-VP-RAR transgene construct. (B) Bright field image of heat-shocked embryos. (C) GFP expression is only seen in Tg(hsp70l:EGFP-VP-RAR) ci104 embryos (right) after heat-shock. |
|
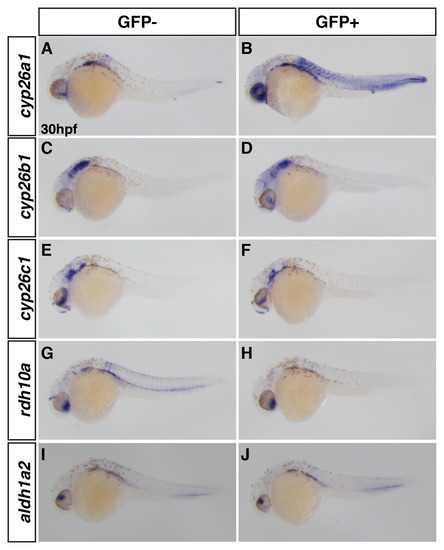
Induction of VP-RAR expression using the Tg(hsp70l:EGFP-VP-RAR) line promotes cyp26a1 expression at later stages. (A,B) Wholemount ISH shows cyp26a1 expression is increased after heat-shock at 26 hpf. (C–F) cyp26b1 is modestly increased after heat-shock at 26 hpf in the head, while cyp26c1 is unchanged. (G–J) Rdh10a expression was decreased after heat-shock at 26 hpf, but aldh1a2 expression was overtly unchanged. GFP- denotes non-transgenic siblings, GFP+ denotes embryos with the hsp70l:EGFP-VP-RAR transgene. |
|
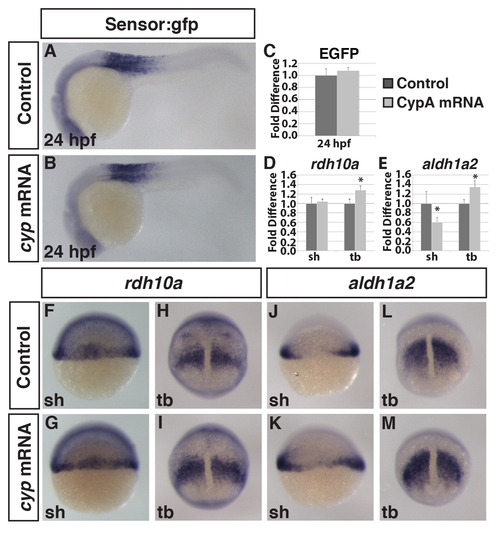
Injection of cyp26a1 mRNA is not sufficient to induce a dramatic loss of RA signaling. (A,B) ISH of GFP expression in Tg(β-actin:GDBD-RLBD);(UAS:EGFP) embryos injected with cyp26a1 (cyp) mRNA show no difference in EGFP. (C) qPCR for EGFP in Tg(β-actin:GDBD-RLBD);(UAS:EGFP) embryos show no difference in EGFP expression between Cyp26a1 mRNA compared to WT siblings. (D,E) Quantification of (F–M) by RT-qPCR shows that both rdh10a and aldh1a2 are increased by tailbud. (F,G,H,I) ISH for rdh10a shows embryos injected with cyp26a1 mRNA have no difference at shield stage but are increased by tailbud. (J,K,L,M) ISH for aldh1a2 shows cyp mRNA injection has no effect at shield stage but leads to an increase in aldh1a2 by tailbud. Asterisks indicate statistically different from controls (p<0.05) using a Student′s t-test. |
|
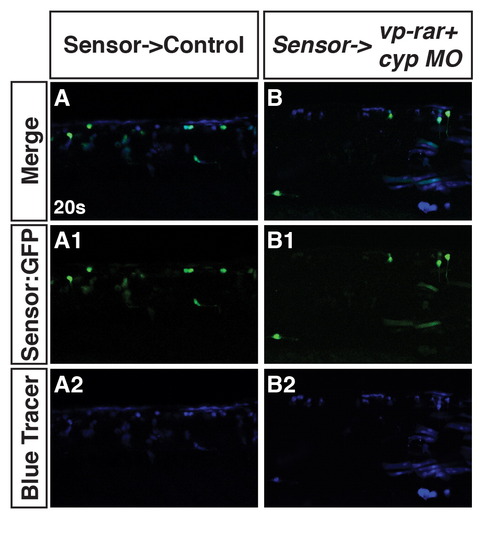
Cyp26a1 is required for the non-cell autonomous loss of RA signaling in vp-rar expressing embryos. (A–A2, B–B2) Tg(β-actin:GDBD-RLBD);(UAS:EGFP) donor cells transplanted into WT hosts or Cyp26a1 deficient hosts injected with vp-rar mRNA have bright EGFP expression in the trunk. |
|
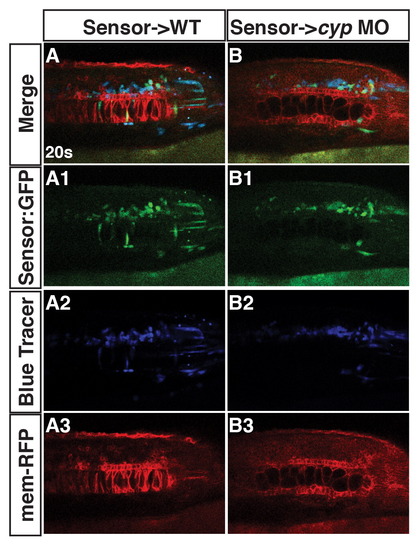
Cyp26a1 deficiency does not alter the ability of RA sensor cells to respond. (A1-3, B1-3) Tg(β-actin:GDBD-RLBD);(UAS:EGFP) donor cells into either WT or Cyp26a1 deficient hosts injected with mrfp mRNA have similar levels of GFP expression in the notochord and spinal cord compared to WT siblings. |
|
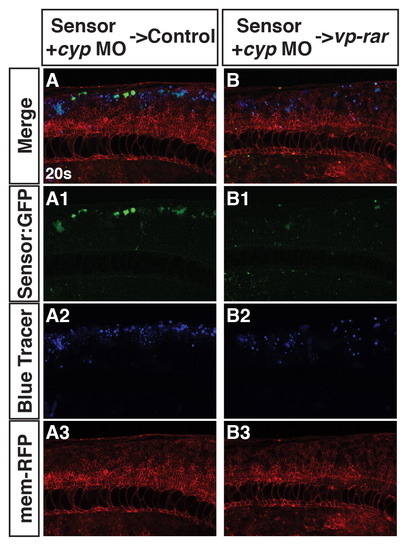
Cell non-autonomous Induction of Cyp26a1 overexpression is not responsible for a loss of RA signaling. (A–A3) Cyp26a1 deficient Tg(β-actin:GDBD-RLBD);(UAS:EGFP) donor cells transplanted into WT hosts have bright EGFP expression. (B–B3) Cyp26a1 deficient Tg(β-actin:GDBD-RLBD);(UAS:EGFP) donor cells transplanted into vp-rar mRNA injected hosts have dampened EGFP expression. |
Reprinted from Developmental Biology, 405(1), Rydeen, A., Voisin, N., D'Aniello, E., Ravisankar, P., Devignes, C.S., Waxman, J.S., Excessive feedback of Cyp26a1 promotes cell non-autonomous loss of retinoic acid signaling, 47-55, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.