- Title
-
Sox10-dependent neural crest origin of olfactory microvillous neurons in zebrafish
- Authors
- Saxena, A., Peng, B.N., and Bronner, M.E.
- Source
- Full text @ Elife
|
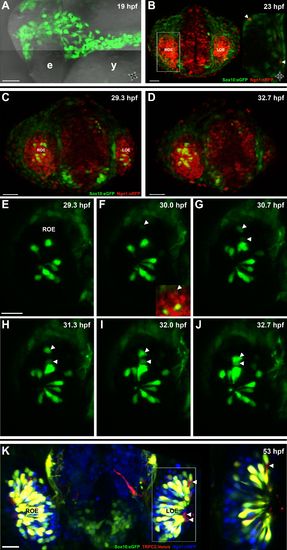
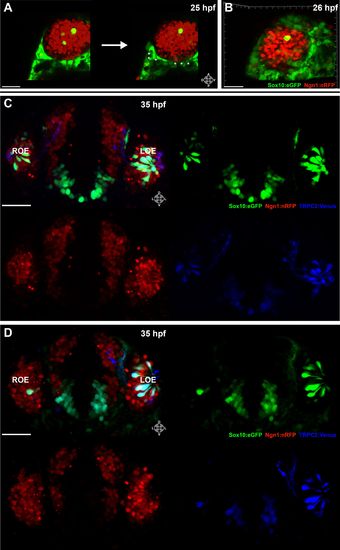
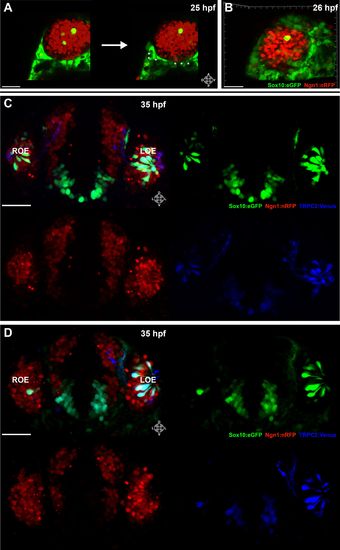
Sox10:eGFP+/Ngn1:nRFP+ cells migrate and differentiate into microvillous neurons. EXPRESSION / LABELING:
|
|
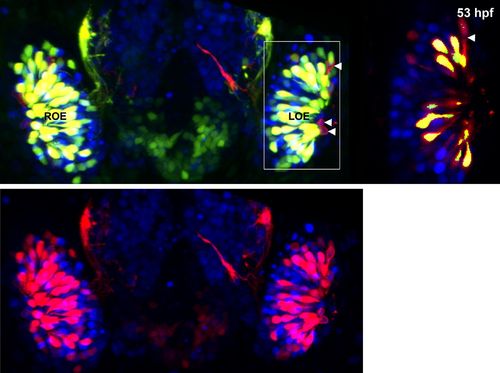
Panel (K) from Figure 1 with Sox10:eGFP (green) channel removed (bottom) to better illustrate the large population of ciliated neurons present basally that are not Sox10:eGFP+ but are Ngn1:nRFP+ (blue). High magnification inset (right) has Sox10:eGFP signal artificially underexposed and decreased to better view the colocalization with membrane TRPC2:Venus expression. |
|
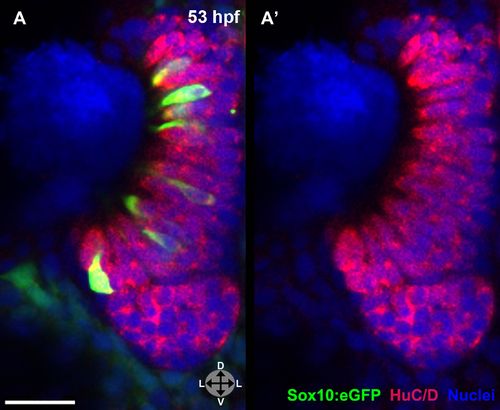
(A) and (A′) Sox10:eGFP+ microvillous neurons in fixed embryos stained with anti-GFP antibody colocalize with anti-HuC/D antibody staining at 53 hpf, confirming post-mitotic neuronal identity. Sox10:eGFP: green; HuC/D: red; nuclear stain: blue. Orientation arrows: D: dorsal; V:ventral; L: lateral. z = 2.5 μm. Scale bar: 20 μm. |
|
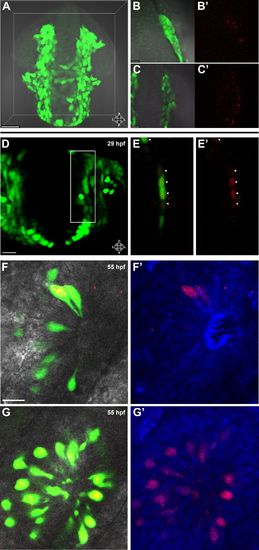
Lineage tracing by photoconversion demonstrates neural crest origin of microvillous neurons. (A) Nuclear Dendra2 (low level green) was photoconverted in Sox10:eGFP+ (bright green) neural crest cells at 14–16 hpf (10–14 somite stage) in live embryos. (B)–(C′) Representative example z-planes show unilateral photoconversion of a few cells (B and B′) or a large number of cells (C and C′). (B and C) show ubiquitous Dendra2 (low level green) and Sox10:eGFP (bright green), and (B′ and C′) show photoconverted Dendra2 (red). (D) Photoconverted cells are visible in the contralateral nasal cavity at 29 hpf; (E and E′) show a 2.5-μm-thick z-plane slice of the boxed area with photoconverted cells (red, arrowheads). (F and F′) At 55 hpf, photoconversion of a small number of cells (B and B′) results in a subset of Sox10:eGFP+ microvillous neurons being labeled by photoconverted Dendra2 on the contralateral side. (G and G′) Large-scale photoconversion (C and C′) labels most Sox10:eGFP+ microvillous neurons. Directly adjacent ciliated neurons are never labeled. (F–G′) z = 2.5 μm; Sox10:eGFP: green; Dendra2Green: blue; Dendra2Red: red; histology in brightfield. Orientation arrows: A: anterior; P: posterior; D: dorsal; V: ventral; L: lateral. Scale bars: 50 μm (A); 20 μm (B–D); 10 μm (F–G′). See alsoFigure 2—figure supplement 1. |
|
Shown are a few 55 hpf microvillous neurons that are Sox10:eGFP negative (arrowheads) and were not photoconverted, suggesting a possible non-neural crest origin. (A) Immediately before and after ablation of Sox10:eGFP+/Ngn1:nRFP+ cells in the right nasal cavity in live embryos; example z-plane slice shows six regions of ablation (asterisks). (B) 3D z-stack post-ablation of several z-planes with total of e20 Sox10:eGFP+/Ngn1:nRFP+ ablated cells. (C) and (D) 35 hpf embryos show a significant decrease in the number of Sox10:eGFP+/TRPC2:Venus+ microvillous neurons within the right olfactory epithelium as compared to the unablated left side in both small (C) and large (D) ablation experiments. Directly adjacent ciliated neurons are only marginally affected. Sox10:eGFP: green; Ngn1:nRFP: red; TRPC2:Venus: blue. LOE: left olfactory epithelium; ROE: right olfactory epithelium. Orientation arrows: A: anterior; P: posterior; D: dorsal; V: ventral; L: lateral. Scale bars: 30 μm (A and B); 40 μm (C and D). |
|
Laser ablation of neural crest in the nasal cavity inhibits microvillous neurogenesis. (A) Immediately before and after ablation of Sox10:eGFP+/Ngn1:nRFP+ cells in the right nasal cavity in live embryos; example z-plane slice shows six regions of ablation (asterisks). (B) 3D z-stack post-ablation of several z-planes with total of e20 Sox10:eGFP+/Ngn1:nRFP+ ablated cells. (C) and (D) 35 hpf embryos show a significant decrease in the number of Sox10:eGFP+/TRPC2:Venus+ microvillous neurons within the right olfactory epithelium as compared to the unablated left side in both small (C) and large (D) ablation experiments. Directly adjacent ciliated neurons are only marginally affected. Sox10:eGFP: green; Ngn1:nRFP: red; TRPC2:Venus: blue. LOE: left olfactory epithelium; ROE: right olfactory epithelium. Orientation arrows: A: anterior; P: posterior; D: dorsal; V: ventral; L: lateral. Scale bars: 30 μm (A and B); 40 μm (C and D). |
|
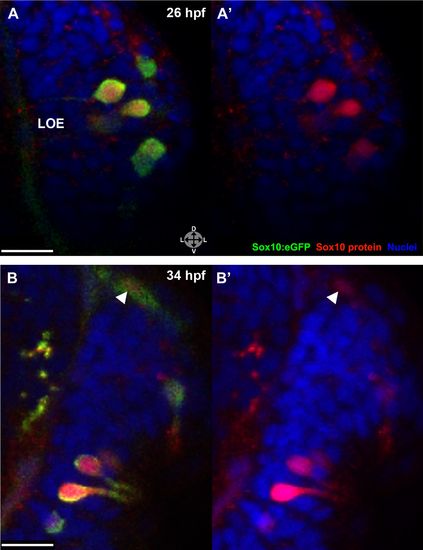
Sox10 protein is preferentially upregulated in neural crest cells that differentiate into microvillous neurons. (A) Immediately before and after ablation of Sox10:eGFP+/Ngn1:nRFP+ cells in the right nasal cavity in live embryos; example z-plane slice shows six regions of ablation (asterisks). (B) 3D z-stack post-ablation of several z-planes with total of e20 Sox10:eGFP+/Ngn1:nRFP+ ablated cells. (C) and (D) 35 hpf embryos show a significant decrease in the number of Sox10:eGFP+/TRPC2:Venus+ microvillous neurons within the right olfactory epithelium as compared to the unablated left side in both small (C) and large (D) ablation experiments. Directly adjacent ciliated neurons are only marginally affected. Sox10:eGFP: green; Ngn1:nRFP: red; TRPC2:Venus: blue. LOE: left olfactory epithelium; ROE: right olfactory epithelium. Orientation arrows: A: anterior; P: posterior; D: dorsal; V: ventral; L: lateral. Scale bars: 30 μm (A and B); 40 μm (C and D). |
|
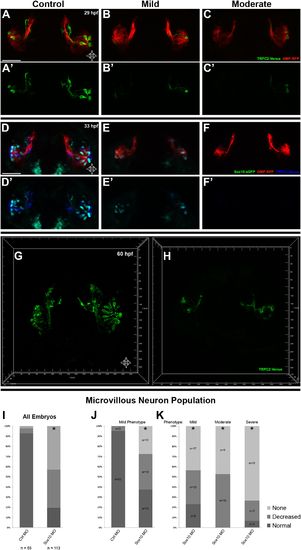
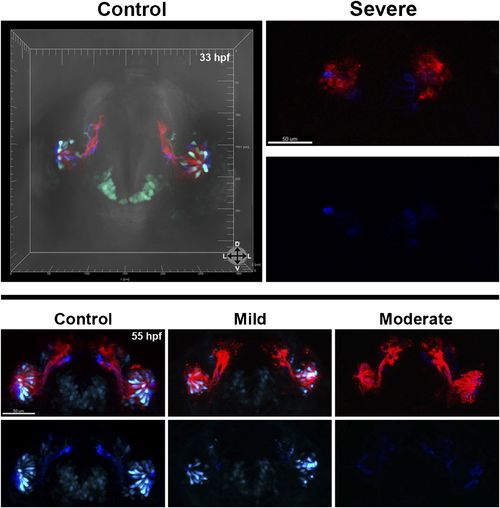
Morpholino knockdown of Sox10 selectively inhibits microvillous neurogenesis. (A) and (A′) Control morpholino-injected live embryos at 29 hpf have ciliated (red) and microvillous (green) neurons. (B)–(C′) Embryos with mild and moderate phenotypes (assayed histologically) display only slight changes in ciliated neurons, whereas microvillous neurons are significantly decreased in number and organization. (D)–(F′) Similar results are seen at 33 hpf, with almost no Sox10:eGFP+ (green) cells becoming microvillous (blue) neurons. Ciliated (red) neurons remain relatively unaffected. (G) and (H) Antibody staining with anti-GFP against TRPC2:Venus in fixed embryos demonstrates a persistent decrease in the number of microvillous cells and disorganization at 60 hpf in Sox10 morpholino-treated embryos (H) in comparison to control embryos (G). All images were captured at identical settings to facilitate direct comparison of control and experimental embryos. (I)–(K) All embryos (I) were divided into ‘high pigmentation′ (J) or ‘low pigmentation′ (K) to roughly correlate Sox10 levels (higher and lower, respectively) with degree of olfactory phenotype. As expected, all control morpholino-treated embryos have high pigmentation, and all Sox10 morpholino-treated embryos with high pigmentation have only a mild phenotype. In all cases (J and K), Sox10 morpholino treatment results in most embryos having either no microvillous neurons or a decreased number. Ratios correlate with degrees of pigmentation and phenotype. (A–C′, G, and H) TRPC2:Venus: green; OMP:RFP: red. (D–F′) Sox10:eGFP: green; OMP:RFP: red; TRPC2:Venus: blue. Orientation arrows: D: dorsal; V: ventral; L: lateral. Scale bars: 50 μm. *p<0.001. See also Figure 5—figure supplement 1. |
|
At 33 hpf, severe phenotype embryos (top right) have major malformations in both microvillous and ciliated neurons and often perish, likely reflecting non-specific effects. At 55 hpf (bottom) are shown the same embryos as in Figure 5 (D–F′). At 55 hpf (bottom) are shown the same embryos as in Figure 5 (D–F′). While there is some recovery of microvillous neurons in ‘mild′ embryos, their numbers remain decreased in comparison to controls. ‘Moderate′ embryos have near complete lack of neural crest-derived microvillous neurons, whereas ciliated neurons are present and project to the olfactory bulb. |
|
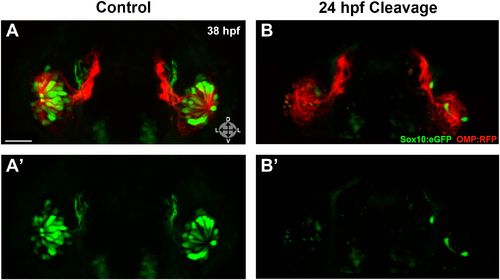
Photo-morpholino knockdown of Sox10 demonstrates its necessity during the ingression/differentiation process. (A) and (A′) Control (antisense morpholino + sense photo-morpholino injected but not photocleaved) live embryos have robust numbers of ciliated (red) and neural crest–derived microvillous (green) neurons at 38 hpf. In contrast, identically injected embryos subjected to photocleavage at 24 hpf go on to develop ciliated neurons but significantly lack microvillous neurons at 38 hpf (B and B′) in comparison to control embryos. Sox10:eGFP: green; OMP:RFP: red. Orientation arrows: D: dorsal; V: ventral; L: lateral. Scale bars: 30 μm. See also Figure 6—figure supplement 1. |
|
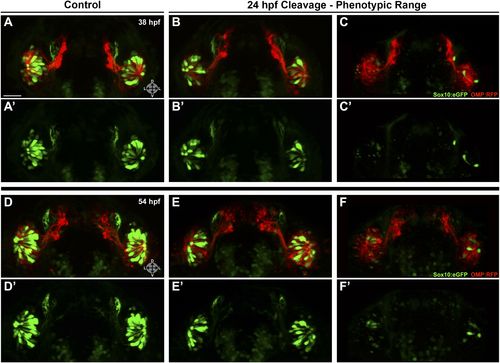
Shown are more details of the phenotype in Figure 6: (A and A′) control (antisense morpholino + sense photo-morpholino injected but not photocleaved) live embryos have robust numbers of ciliated (red) and neural crest–derived microvillous (green) neurons at 38 hpf. In contrast, identically injected embryos subjected to photocleavage at 24 hpf go on to develop ciliated neurons but have slight (B and B′) or, much more commonly, large (C and C′) decreases in microvillous neuron numbers and organization in comparison to control embryos. Photocleavage may have been less efficient in some embryos, resulting in a range of phenotypic severity. (D–F′) similar results are seen in the same embryos at 54 hpf, now with an even greater difference in microvillous neuron numbers between control and cleaved embryos. Sox10:eGFP: green; OMP:RFP: red. Orientation arrows: D: dorsal; V: ventral; L: lateral. Scale bars: 30 μm. |