- Title
-
Fibroblast growth factor receptor 2c signaling is required for intestinal cell differentiation in zebrafish
- Authors
- Liu, D.W., Tsai, S.M., Lin, B.F., Jiang, Y.J., and Wang, W.P.
- Source
- Full text @ PLoS One
|
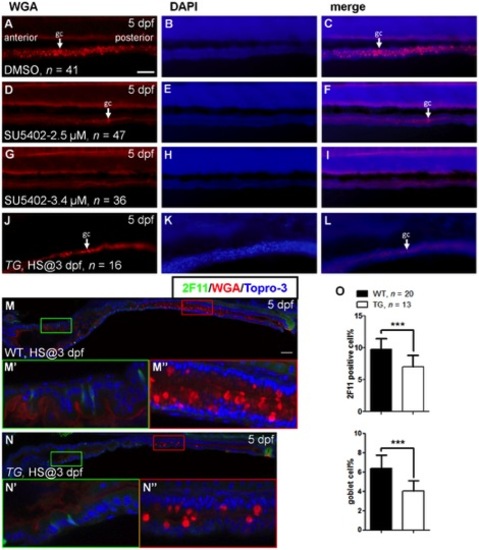
Effects on cell differentiation after inhibition of Fgf signaling. |
|
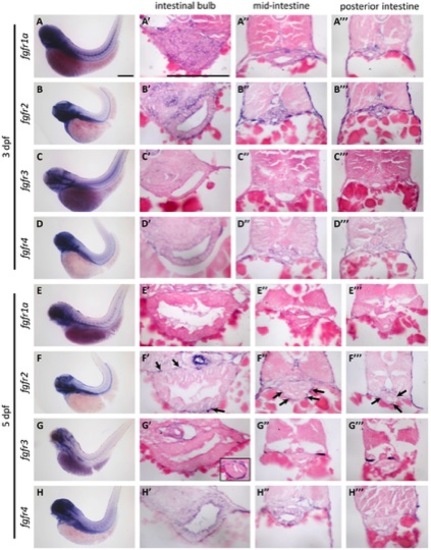
The expression pattern of zebrafish fgfr genes. EXPRESSION / LABELING:
|
|
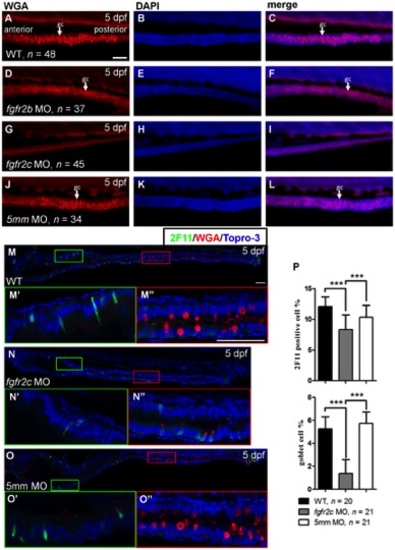
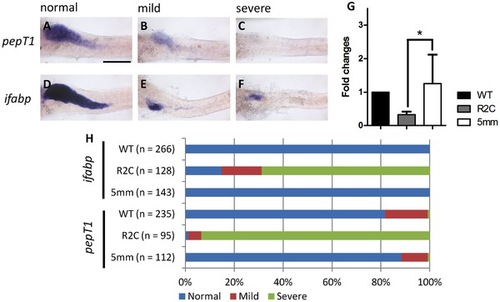
The secretory cell differentiation of fgfr2c morphants. |
|
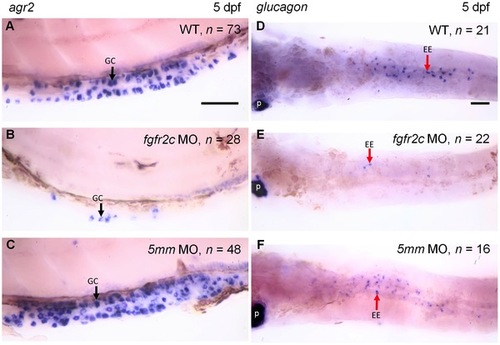
The expression of agr2 and glucagon in fgfr2c morphants. |
|
Absorptive cell differentiation in fgfr2c morphants. |
|
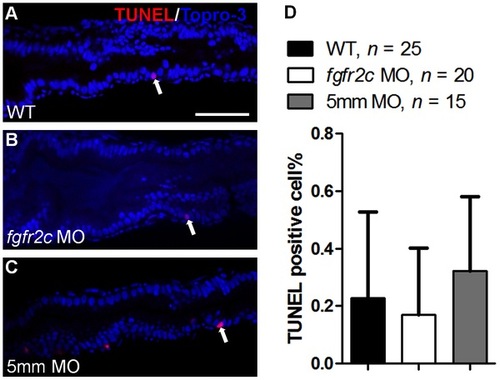
Cell death in fgfr2c morphants. TUNEL assay was performed on (A) WT embryos, (B) fgfr2c morphants, and (C) fgfr2c-5 mm morphants at 5 dpf. TUNEL-positive cells (red) were detected in developing gut (indicated by white arrows). Topro-3 was used for nuclear counter staining (blue). (D) The bar charts show the percentages of TUNEL-positive cells in WT embryos, and fgfr2c and fgfr2c-5 mm morphants. Error bars indicate SD. Scale bar = 50 μm. |
|
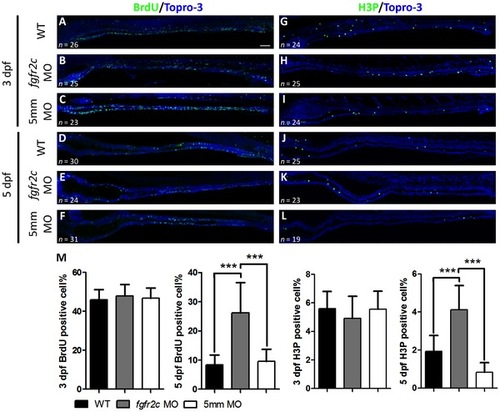
Cell proliferation in fgfr2c morphants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
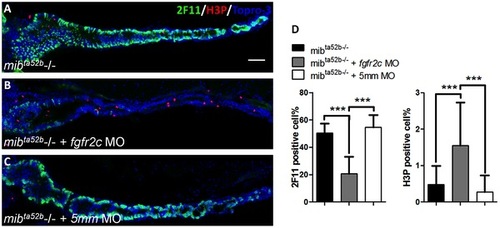
The secretory cell differentiation of mibta52b mutants after injection with fgfr2c morpholino. EXPRESSION / LABELING:
|
|
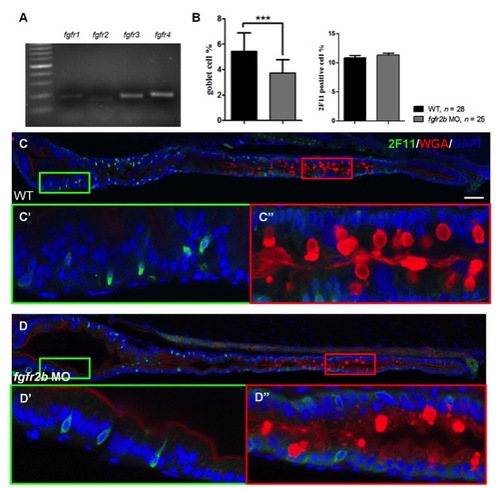
The expression of fgfrs and the secretory cell differentiation of fgfr2b morphants. (A) fgfr1a, fgfr2, fgfr3 and fgfr4 gene were analyzed in 5 dpf zebrafish gut tissue by RT-PCR. (C) WT embryos and (D) fgfr2b morphants were double labeled using 2F11 antibody and WGA. The magnified image shows (C′–D′) enteroendocrine cells and (C′′–D′′) goblet cells. DAPI was used for nuclear counter staining (blue). (B) The bar charts show the percentages of 2F11 and WGA positive cells. Error bars indicate SD. Scale bar = 50 μm. |
|
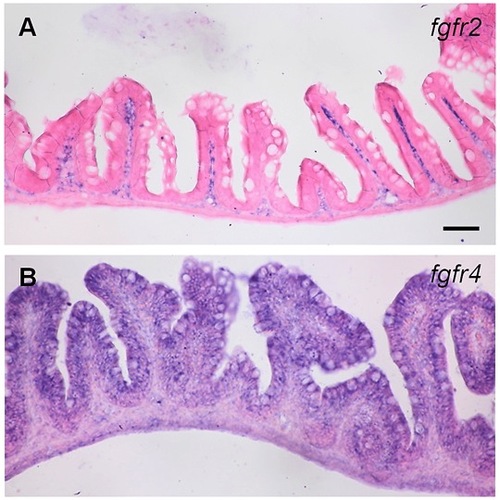
The expression of fgfr2 and fgfr4 in adult zebrafish intestine. Section in situ hybridization was used to analyze the expression of fgfr2 and fgfr4 genes. (A) fgfr2 was detected in the lamina propria, and (B) fgfr4 was expressed mainly in the epithelial layer of the intestine. Scale bar = 50 μm. EXPRESSION / LABELING:
|