- Title
-
Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis
- Authors
- Barrios, A., Poole, R.J., Durbin, L., Brennan, C., Holder, N., and Wilson, S.W.
- Source
- Full text @ Curr. Biol.
|
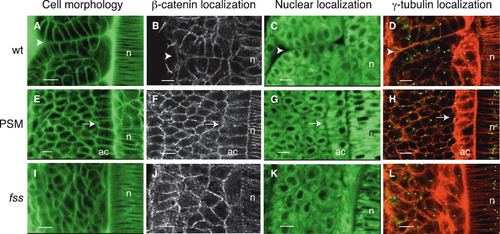
Cells Undergo Mesenchymal-to-Epithelial Transition at Somite Boundaries (A–L) Dorsal views of the left-sided paraxial mesoderm of embryos labeled with Bodipy ceramide (which reveals cell morphology; [A], [E], and [I]) or with Bodipy 505-515 (which reveals nuclear position, [C], [G], and [K]) or immunostained for β-catenin ([B], [F], and [J]) or for γ-tubulin (which labels centrosomes) and stained with phalloidin (which labels actin) ([D], [H], and [L]). Anterior is oriented toward the top. (A–D) Cells at somite boundaries in wild-type embryos. The arrowheads point to the intersomitic boundary. (E–H) Cells in the presomitic mesoderm (PSM) of wild-type embryos. The arrows point to epithelial adaxial cells in which centrosomes are apically localized (H), as also seen in epithelial cells at somite boundaries (D). Centrosomes are randomly positioned in other PSM cells. (I–L) Cells in the somitic mesoderm of fss-/- embryos. n, notochord; ac, adaxial cells. The scale bars represent 10 μm. |
|
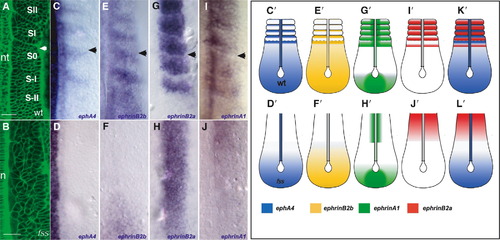
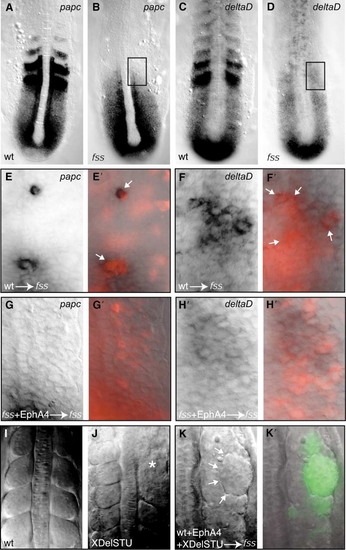
Expression of Eph Family Members in the Paraxial Mesoderm of Wild-Type and fss-/- Embryos Dorsal views of the paraxial mesoderm of 8-somite-stage wild-type and 10-somite-stage fss-/- embryos and schematics with anterior oriented toward the top. The arrowheads indicate the position of the most recently formed intersomitic boundary. (A and B) Living wild-type and fss-/- embryos labeled with Bodipy ceramide. In the wild-type embryo, the positions of the last two somites formed (SII, SI), the forming somite (S0), and the two presumptive somites in the PSM (S-I, S-II) are indicated. The arrowhead points to the intersomitic boundary. (C–J) Expression of (C and D) ephA4, (E and F) ephrin-B2b, (G and H) ephrin-B2a, and (I and J) ephrin-A1 in wild-type (top row) and fss mutant (bottom row) embryos in the region of the paraxial mesoderm shown in (A) and (B). (C′–J′, K, and L) Schematics summarizing expression of Eph family members in the paraxial mesoderm of wild-type and fss-/- embryos. nt, neural tube; n, notochord. The scale bars represent 30 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
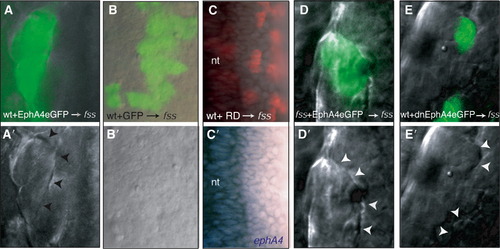
Eph/Ephrin Signaling Restores Morphologically Distinct Boundaries in fss-/- Embryos (A–E and A′–E′) (A–E) DIC and fluorescence overlays and (A′–E′) DIC images of the paraxial mesoderm of fss-/- hosts into which wild-type (wt) or fss-/- cells expressing various GFP-tagged reagents (green labeling in [A], [B], [D], and [E]) or containing rhodamine dextran (RD, red labeling in [C]) have been transplanted. Reagents are indicated at the bottom of the panels. (C′) ephA4 expression (blue) is absent from the transplanted cells. The arrowheads point to morphologically distinct boundaries formed at the interface between donor and host cells. nt, neural tube. |
|
Boundaries Restored by Eph/Ephrin Signaling Are Maintained during Muscle Differentiation (A–E) Lateral views of (A) wild-type and (B–E) fss-/- somitic muscles immunostained for myosin (green). Anterior is oriented toward the left. (C), (D), and (E) show transplanted (C and E) wild-type or (D) fss-/- cells (red) expressing (C and D) full-length or (E) truncated, dominant-negative EphA4. The arrowheads point at morphologically distinct furrows. The arrows point at fss-/- muscle fibers that span adjacent segments. |
|
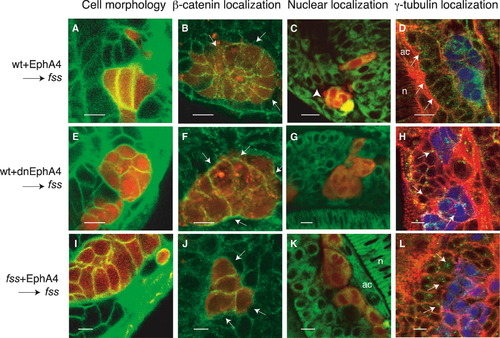
Eph/Ephrin Signaling Rescues Epithelialization of Cells at Morphologically Distinct Boundaries (A–L) (A, E, and I) Confocal images showing Bodipy ceramide-labeled somitic mesoderm of fss-/- host embryos (green) containing rhodamine dextran-labeled donor cells (red). (B, F, and J) Confocal images of β-catenin immunolocalization (green) in transplanted rhodamine dextran-labeled cells (red) in fss-/- host embryos. The arrows point to the basal surfaces of the transplanted cells at the interface at which morphologically distinct boundaries form (visible with DIC optics, not shown). In (B) and (J), β-catenin is reduced on the basal surfaces of these cells. (C, G, and K) Confocal images showing Bodipy 505-515-labeled somitic mesoderm of fss-/- host embryos (green) containing rhodamine dextran-labeled donor cells (red). The white arrowhead points to nuclei localized at the basal pole of host fss-/- cells, adjacent to the boundaries created between donor and host cells. (D, H, and L) Confocal images showing phalloidin-labeled somitic mesoderm of fss-/- host embryos (red) containing CFP-labeled donor cells (blue) and immunostained for γ-tubulin (green). The white arrows point to centrosomes. These are localized at the apical pole of host fss-/- cells adjacent to the boundary created between donor and host cells in (D) but are randomly positioned in (H) and (L). Donor cells are wild-type cells expressing (A–D) full-length or (E–H) truncated, dominant-negative EphA4 or fss-/- cells expressing (I–L) full-length EphA4. n, notochord; ac, adaxial cells. The scale bars represent 10 μm. |
|
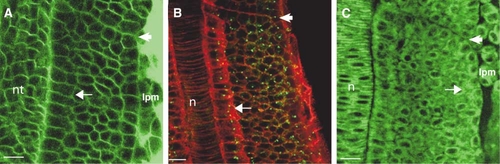
Cells within the Core of the PSM Are Mesenchymal, and Epithelialization of Somite Boundary Cells Occurs Concomitantly with Boundary Formation (A–C) Dorsal views of the PSM of wild-type embryos; anterior is oriented toward the top. Embryos have been stained with (A) Bodipy ceramide or (B) phalloidin (red) or (C) Bodipy 505-515. In (B), the embryo has been immunostained for γ-tubulin (green) to show centrosomes. The arrows point to presomitic cells that have epithelial morphology adjacent to the (A) neural tube, the (B) notochord, and the (C) lateral plate mesoderm. Other cells in the PSM have mesenchymal morphology. The arrowheads indicate the most recently formed or forming intersomitic boundary in each panel. Cells on either side of this boundary only begin to acquire epithelial morphology concomitant with boundary formation. Full maturation of epithelial morphology (see Figures 1A–1D in the main text) occurs after boundary furrow formation. lpm, lateral plate mesoderm; nt, neural tube; n, notochord. The scale bar represents 15 μm. |
|
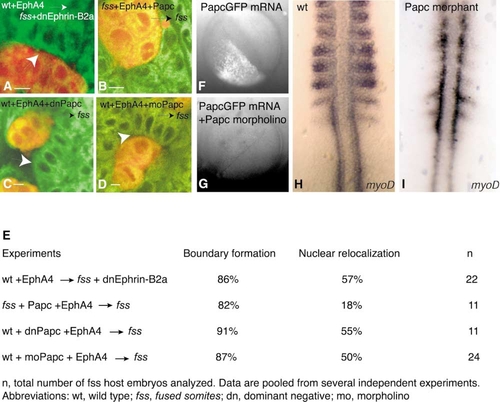
EphA4 Is Unlikely to Induce Boundary Formation by Restoring “Anterior Segmental Identity” to Donor Cells |
|
Epithelialization of Host Cells May Be Independent of Ephrin Reverse Signaling and Independent of Cell-Nonautonomous Activity of Papc |
|
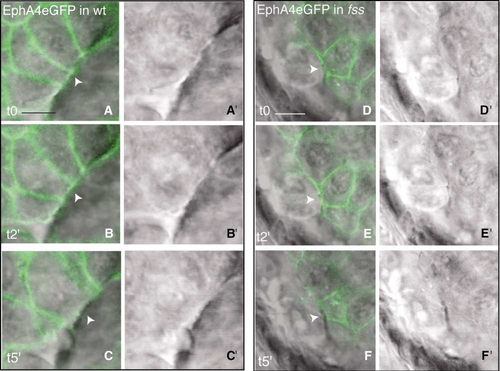
EphA4 Is Removed from the Basal Cell Membrane during Somite Boundary Formation |