- Title
-
Genetic and chemical modulation of spastin-dependent axon outgrowth in zebrafish embryos indicates a role for impaired microtubule dynamics in hereditary spastic paraplegia
- Authors
- Butler, R., Wood, J.D., Landers, J.A., and Cunliffe, V.T.
- Source
- Full text @ Dis. Model. Mech.
|
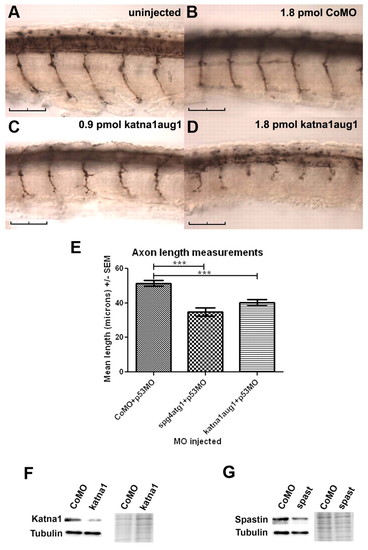
Morpholino-mediated knockdown of katna1 function with a translation-blocking (katna1aug1) morpholino inhibits spinal motor axon outgrowth. Embryos were injected with 1.8 pmol of control (CoMO) (B), 0.9 pmol of katna1aug1 (C) or 1.8 pmol of katna1aug1 morpholino (D), fixed at 28 hpf, and then immunostained with znp-1. An uninjected control is shown in A. The effects of katna1 and spast translation-blocking morpholinos on spinal motor axon outgrowth are independent of p53 (E). Axons were measured in embryos injected with 1.0 pmol CoMO + 1.6 pmol p53-specific morpholino (p53MO) (n=20), 0.6 pmol spg4atg1 + 1.2 pmol p53MO (n=21), or 1.0 pmol katna1aug1 + 1.6 pmol p53MO (n=17). Statistical significance was determined using ANOVA with Bonferroni’s multiple comparison test. ***P<0.001. (F) Immunoblots for katna1 and tubulin demonstrating that katna1aug1-injected embryos have reduced levels of katna1 protein compared with CoMO-injected embryos. (G) Immunoblots for spastin and tubulin demonstrating that spg4atg1-injected embryos show reduced levels of spastin protein compared with CoMO-injected embryos. The right panel in F and G is a section from an equivalently loaded Coomassie-blue-stained gel to demonstrate relative loadings. Scale bars: 50 μm. |
|
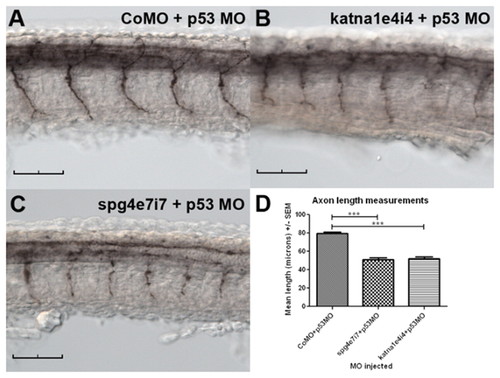
A katna1 splice-blocking (katna1e4i4) morpholino inhibits spinal motor axon outgrowth in a p53-independent manner. Embryos were injected with 1.0 pmol CoMO + 1.6 pmol p53MO (A), 0.8 pmol katna1e4i4 + 1.6 pmol p53MO (B) or 1.0 pmol spg4e7i7 + 1.6 pmol p53MO (C), fixed at 28 hpf, and then immunostained with znp-1. Both katna1 and spast splice-blocking morpholinos inhibit spinal motor axon outgrowth in a p53-independent manner (D). CoMO + p53MO (n=25), spg4e7i7 + p53MO (n=35), katna1e4i4 (n=34); statistical significance was determined using ANOVA with Bonferroni’s multiple comparison test. ***P<0.001. Scale bars: 50 μm. |
|
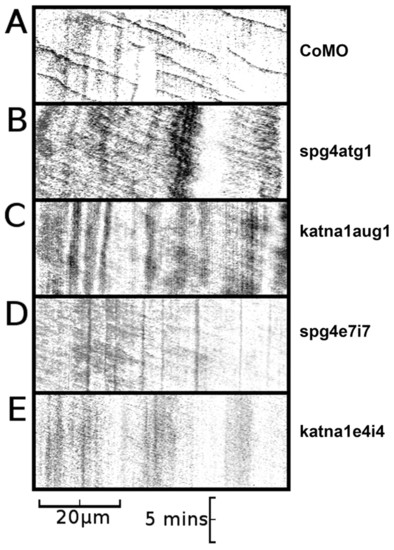
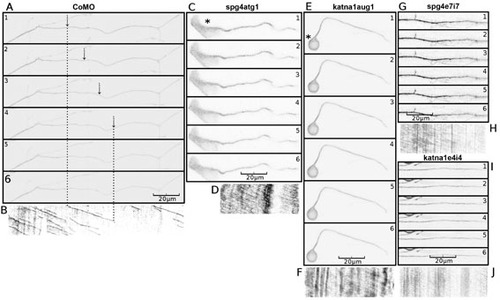
Axons in spast and katna1 morphants lack discrete EB3-GFP puncta. (A) Kymograph of an axon made from a 10-minute time-lapse recording of a neuron from a CoMO-injected embryo, demonstrating anterograde movement of discrete comet-like puncta of EB3-GFP fluorescence (CoMO, n=21; spg4CoMO, n=12). (B) Kymograph of an axon made from a 10-minute time-lapse recording of a neuron from a spg4atg1 (0.6 pmol)-injected embryo, demonstrating weak anterograde movement of diffuse GFP fluorescence in the absence of discrete comet-like puncta of EB3-GFP fluorescence (n=13). (C) Kymograph of an axon made from a 10-minute time-lapse recording of a neuron from a katna1aug1 (1.8 pmol)-injected embryo, again demonstrating weak anterograde movement of diffuse GFP fluorescence in the absence of discrete puncta of EB3-GFP fluorescence (n=10). Equivalent results were obtained with the spg4e7i7 [1.2 pmol; n=6; (D)] and katna1e4i4 [1.2 pmol; n=6; (E)] morpholinos. Horizontal scale bar: 20 µm, vertical scale bar: 5 minutes. A series of individual frames from each of these time-lapse recordings is shown in supplementary material Fig. S4. See supplementary material Movies 2-6. PHENOTYPE:
|
|
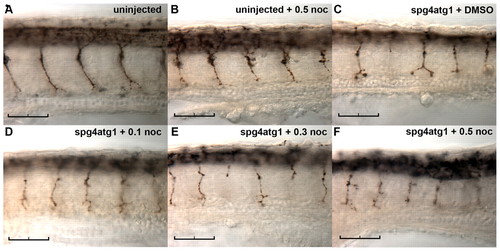
Nocodazole does not ameliorate the effect of reduced spast activity on spinal motor axon outgrowth. Embryos were microinjected with 0.4 pmol of spg4atg1 morpholino and continuously exposed to nocodazole at the concentrations indicated from 24 hpf to 30 hpf, prior to fixation and immunostaining with znp-1. Representative images are shown of an untreated uninjected embryo (A); an uninjected embryo treated with 0.5 μg/ml nocodazole (B); a spast-morphant embryo treated with DMSO vehicle (C); a spast-morphant embryo treated with 0.1 μg/ml nocodazole (D); a spast-morphant embryo treated with 0.3 μg/ml nocodazole (E); and a spast-morphant embryo treated with 0.5 µg/ml nocodazole (F). Scale bars: 50 μm. |
|
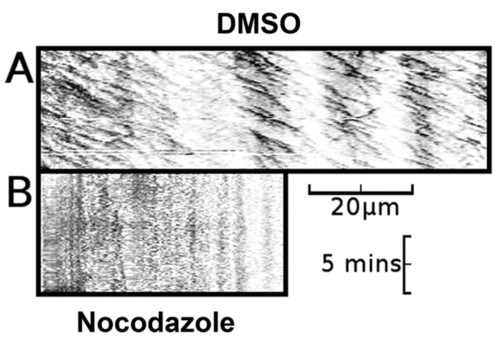
Nocodazole-treated embryos lack both EB3-GFP puncta and axonal movement of EB3-GFP. (A) Kymograph of an axon made from a 10-minute time-lapse recording of a neuron from a DMSO-vehicle-treated embryo, demonstrating predominantly anterograde movement of discrete puncta of EB3-GFP fluorescence (n=16). (B) Kymograph of an axon made from a 10-minute time-lapse recording of a neuron from a nocodazole-treated embryo, demonstrating absence of axonal movement of EB3-GFP fluorescence (n=10). Horizontal scale bar: 20 μm, vertical scale bar: 5 minutes. See supplementary material Movies 7 and 8. |
|
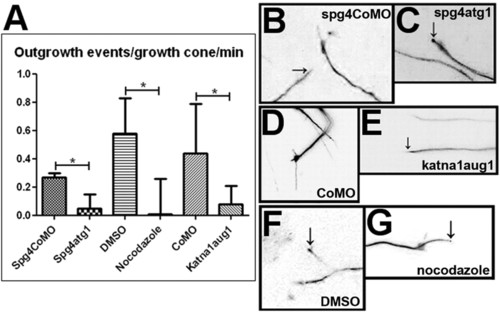
Disruption of microtubule dynamics reduces growth cone activity in the zebrafish embryonic CNS. An outgrowth event was scored as a continuous period of growth of a single process from the distal end of an axon. Scoring was carried out on 10-minute time-lapse recordings and the mean number of outgrowth events per growth cone was calculated. (A) The number of outgrowth events occurring per growth cone per minute is reduced by knockdown of spast (P=0.0179), knockdown of katna1 (P=0.025) and treatment with nocodazole (P=0.0155). Values were calculated using a two-tailed Student’s t-test assuming unequal variance. (B–G) Frames taken from 10-minute time-lapse recordings of the distal part of EB3-GFP-expressing neurons in live zebrafish embryos. When embryos were injected with spastin control morpholino (B), standard control morpholino (D), or treated with DMSO (F), growth cones were highly motile and rapid outgrowth and branching of GFP-labelled processes (arrows in B and F) was observed. When embryos were injected with spast (C) or katna1 (E) morpholinos, or treated with nocodazole (G), growth cone activity was greatly reduced. In some cases, the tips of immobile processes (arrows in C and G) were brightly labelled with an accumulation of EB3-GFP. Scale bars: 10 μm. See supplementary material Movies 9-14. PHENOTYPE:
|
|
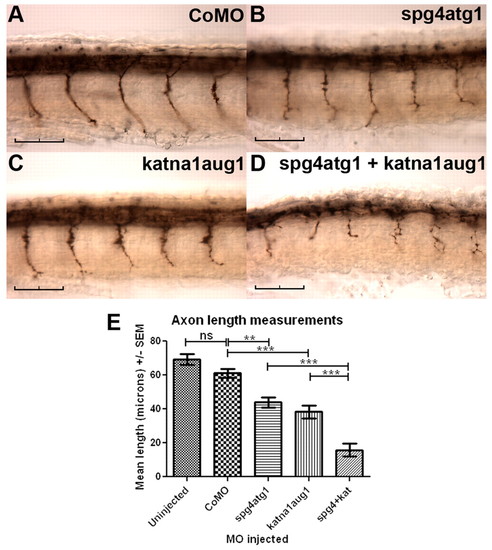
Simultaneous knockdown of katna1 and spast enhances phenotypic severity. Immunostaining with znp-1 demonstrates that embryos co-injected with 0.3 pmol of spg4atg1 and 0.9 pmol of katna1aug1 morpholinos showed far more severe defects in spinal motor axon outgrowth (D) than embryos injected with either 0.3 pmol of spg4atg1 (B) or 0.9 pmol of katna1aug1 (C) alone. (A) Control embryo injected with 1.2 pmol of a control morpholino. (E) Quantification of mean spinal motor axon length in morpholino-injected embryos. Statistical significance was determined using ANOVA with Bonferroni’s multiple comparison test: ns, not significant; ** P<0.01; ***P<0.001. Scale bars: 50 μm. |
|
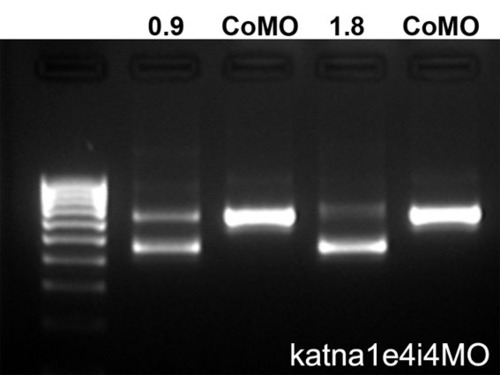
RT-PCR analysis of embryos injected with a katna1 splice-blocking morpholino (katna1e4i4). Embryos were injected with 0.9 pmol or 1.8 pmol of katna1e4i4 or control morpholino (CoMO). RT-PCR with primers spanning exons 2-6 gave a 646 bp product in CoMO-injected embryos and an additional smaller product consistent with skipping of exon 4 (646-187=459 bp) in katna1e4i4-injected embryos. The increased severity of motor axon outgrowth defects in embryos injected with the higher dose of the morpholino correlated with increased levels of the shorter product. |
|
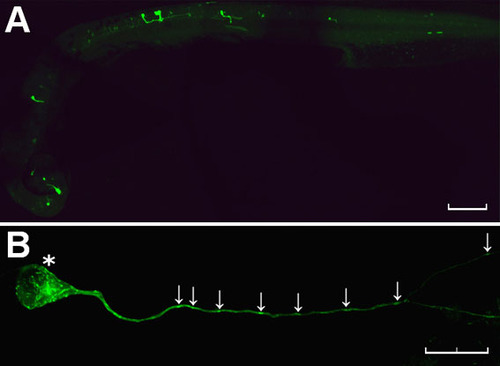
Mosaic expression of NBT:EB3-GFP in a zebrafish embryo. (A) Low-power view of a fixed embryo shows GFP fluorescence in a scattered population of neurons distributed randomly throughout the brain and spinal cord (anterior to left, dorsal top). (B) High-power view of a single EB3-GFP-expressing neuron in the CNS of a fixed zebrafish embryo reveals discrete labelling of puncta in the cell body and throughout the length of the axon (arrows). Scale bars: 100 μm (A) and 20 μm (B). |
|
Anterograde movement of EB3-GFP puncta in a live zebrafish neuron. (A) Single frames taken from 10-minute time-lapse recording of an EB3-GFP-injected embryo (n=15) showing anterograde movement of discrete comet-like puncta of EB3-GFP fluorescence (arrows) away from the cell body (asterisk). (B) Kymograph of axon shown in A. The arrows indicate the anterograde movement of three individual comets, and the dotted lines indicate the extent of movement of one of these structures over a 10-minute period. Scale bar: 20 μm. See supplementary material Movie 1. |
|
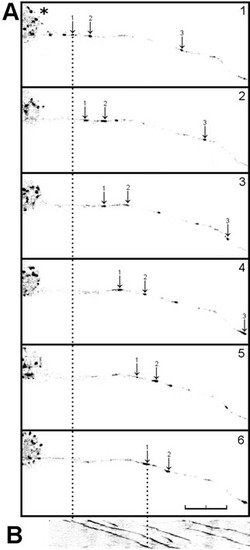
Axons in spast and katna1 morphants lack discrete EB3-GFP puncta. Single frames (A, C, E, G, I) taken from time-lapse recordings used to make the kymographs (B, D, F, H, J) shown in Fig. 3. Embryos were injected with EB3-GFP plasmid DNA and control (A, B), spg4atg1 (C, D), katna1aug1 (E, F), spg4e7i7 (G, H) and katna1e4i4 (I, J) morpholinos. |
|
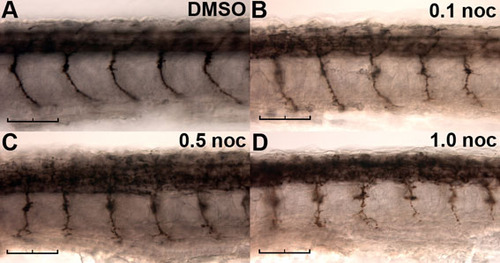
Effect of nocodazole on spinal motor axon outgrowth. Wild-type embryos were continuously exposed to DMSO vehicle (A) or 0.1 (B), 0.5 (C) or 1.0 (D) μg/ml nocodazole from 24 hpf, then fixed at 30 hpf for immunostaining with znp-1. Scale bars: 50 μm. |