- Title
-
Apc1 is required for maintenance of local brain organizers and dorsal midbrain survival
- Authors
- Paridaen, J.T., Danesin, C., Elas, A.T., van de Water, S., Houart, C., and Zivkovic, D.
- Source
- Full text @ Dev. Biol.
|
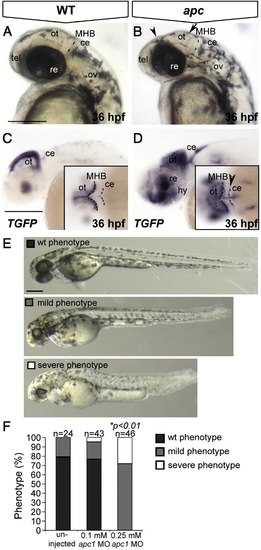
The apcCA50a/CA50a is a zygotic-effect mutation resulting in abnormal brain development and aberrant LEF/β-catenin transcription. Lateral view, anterior to the left. (A) Wild-type and apc mutant embryo (B) at 36 hpf. Note the enlarged otic vesicles, smaller irregular eyes, smaller and flattened optic tectum (arrow) and diencephalon (arrowhead) and less discrete MHB. Dashed line indicates the MHB. (C, D) In wild-type embryos at 36 hpf (C), TGFP is restricted to the dorsal midline and posterior boundary of the optic tectum. In apc mutants (D), TGFP is expanded in the tectum, hypothalamus and cerebellum (arrowhead in inset D). Dashed lines indicate MHB and cerebellum. (E, F) Microinjection of a translation-blocking apc1 morpholino (zfapc1 MO) into progeny of apc+/- carriers results in exacerbated phenotypes. (E) The phenotype was categorized according to these categories: wild-type (black), ‘mild’ - reminiscent of apc zygotic phenotype (gray) and ‘severe’ - apc phenotype with additional reduction of eye size, flattening of somites and reduced embryo length (white). In uninjected embryos, only wild-type and ‘mild’ phenotypes were observed. (F) Graph showing the proportion of embryos that show each phenotype. A portion of the embryos in (E, F) was genotyped. Upon injection of 0.1 mM MO, 29% of apc-/- embryos showed a ‘severe’ phenotype (n = 7). Upon injection of 0.25 mM MO, 75% of apc-/- mutants (n = 8) acquired a ‘severe’ phenotype versus only 11% of heterozygous apc+/- embryos (n = 19) and 14% of wild-types (n = 7). Insets - dorsal view. ce, cerebellum; hy, hypothalamus; MHB, mid–hindbrain boundary; re, retina; ot, optic tectum; ov, otic vesicle; tel, telencephalon. Scale bar 250 μm. |
|
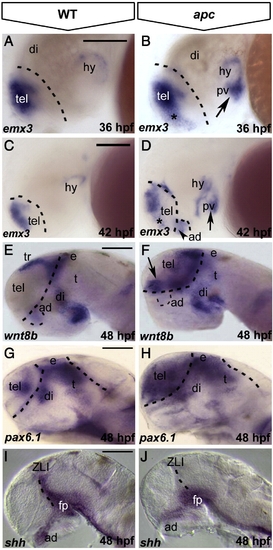
Loss of Apc function leads to mispatterning of the forebrain. Lateral view, anterior to the left. (A–D) As compared to wild-type embryos (A), emx3 expression expands into ventral telencephalon (asterisk) and posterior ventral hypothalamus (arrow) in apc mutants at 36 hpf (B). At 42 hpf (C, D), emx3 further expands into anterior dorsal hypothalamus (dashed line and arrowhead in panel D) and the telencephalic–diencephalic boundary. (E–H) wnt8b (E, F) and pax6.1 (G, H) expand rostrally into the telencephalon and caudally into the thalamus of apc mutants (F, H) at 48 hpf. Dashed line in panels A–H indicates the telencephalic–diencephalic boundary. An additional line in panels G, H indicates the ventral/dorsal midbrain boundary. (I, J) In apc mutants, shh expression is absent from the ZLI and anterior dorsal hypothalamus at 48 hpf (J). Dashed line indicates the ZLI. ad, anterior dorsal hypothalamus; di, diencephalon, e, epithalamus; fp, floorplate; pv, posterior ventral hypothalamus; t, thalamus; ZLI, zona limitans intrathalamica. Scale bar 125 μm. |
|
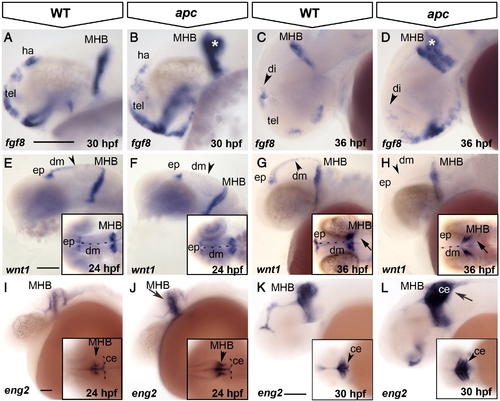
Loss of Apc function compromises the IsO. Lateral view, anterior to the left. (A–D) fgf8 expression expands in the ventral telencephalon and into the cerebellum (asterisk) in apc mutants at 30 hpf (B). At 36 hpf (C, D), fgf8 is reduced in the habenula (arrowhead in panel D). (E–H) At 24 hpf, wnt1 expression is reduced in apc mutants (F) from the epiphysis and dorsal midline (arrowhead in panel F) and entirely absent from the dorsal midline at 36 hpf (H). wnt1 is weakly expanded into the mutant cerebellum (arrow in inset panel H). Inset - dorsal view. Dashed line indicates the midline. (I–L) At 24 hpf, eng2a expression is enhanced at the apc MHB (arrow and arrowhead in panel J; compare to inset in panel I). At 30 hpf (K, L), eng2a is expanded into the cerebellum (arrow in panel L and arrowhead in inset). Inset - dorsal view. Dashed line indicates cerebellum. ce, cerebellum; dm, dorsal midline; ep, epiphysis; ha, habenula; MHB, mid–hindbrain boundary; tel, telencephalon. Scale bar 125 μm. |
|
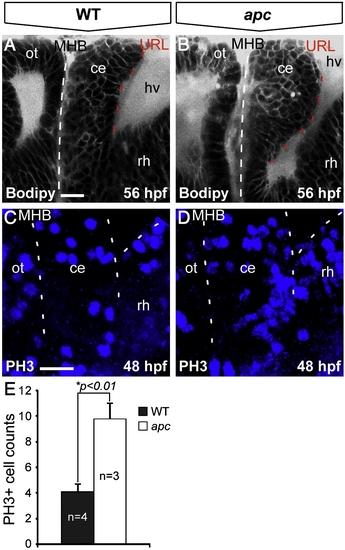
Loss of Apc function results in unscheduled proliferation in the cerebellum. Lateral view, anterior to the left. (A, B) Bodipy vital staining marking cell membranes reveals that the apc cerebellum (B) is enlarged and curves towards the MHB (indicated with dashed line) as compared to wild-type cerebellum (A). (C, D) Phosphohistone-3 (PH3) immunolabeling that marks mitotic cells identifies unscheduled increased proliferation in the mutant cerebellum (D) as compared to wild-types (A). Left dashed line indicates MHB, bifurcating dashed lines on the right indicate boundary between cerebellum and the hindbrain/rhombomere 1. (E) The mutant cerebellum shows a significantly higher number of PH3+ cells as determined by Student′s t-test (*p < 0.01). Error bars represent standard error of the mean (SEM). ce, cerebellum; hv, hindbrain ventricle; MHB, mid–hindbrain boundary; ot, optic tectum; rh, rhombomere1. Scale bar 15 μm. |
|
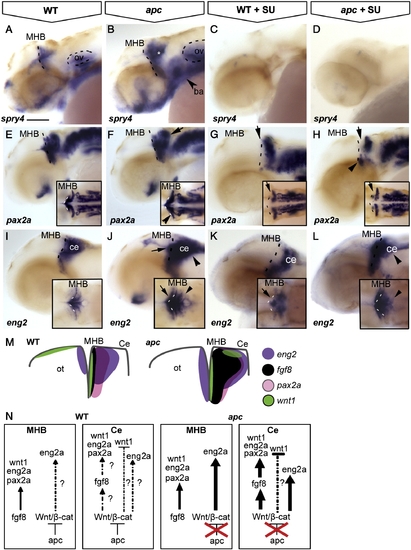
The contribution of Wnt/β-catenin and Fgf8 signalling to maintenance of the IsO. Lateral view, anterior to the left. Embryos at 42 hpf. (A, B) The Fgf8 target spry4 is expanded in the MHB, cerebellum (asterisk) and branchial arches (arrowhead) of apc mutants. (C, D) Upon treatment with 8 and 25 μM SU5402, spry4 is lost from both wild-type and mutant embryos. (E–H) pax2a is expanded laterally at the MHB (arrowhead in inset F) and caudally into the cerebellum of apc mutants (arrow) as compared to wild-types. (G, H) After treatment with 25 μM SU5402, pax2a is abolished from the MHB (arrow) of wild-type embryos (G) and in apc mutants (H). pax2a expression in the ventral MHB of apc mutants appeared refractory to Fgf inhibition (arrowhead in panel H), although pax2a expression is absent from the pons in treated apc mutants. (I–L) In wild-type embryos (I, K), SU5402 treatment caused disruption of eng2a expression at the MHB (arrow). However, in apc mutants, eng2a expression persisted in the MHB upon SU5402 treatment (L). In contrast, eng2a expression in the cerebellum is slightly downregulated (arrowhead in panel L) as compared to untreated mutants (arrowhead in panel J;). Dashed line indicates the MHB. Insets - dorsal view. (M) Schematic view showing expression of regulatory genes in the isthmic region. (N) Model showing regulation of IsO genes by Fgf8 and Wnt/β-catenin. In wild-type embryos, Apc1 expression in the cerebellum mediates restriction of Fgf8 expression by limiting Wnt/β-catenin signalling and thereby preventing caudal expansion of the isthmic markers pax2a, wnt1 and eng2a that are regulated by Fgf8. In apc mutants, the restriction of Wnt/β-catenin signalling is lifted, and overactivated Wnt/β-catenin signalling causes direct caudal expansion into the cerebellum and MHB of eng2a and indirect expansion of pax2a through ectopic Fgf8. wnt1 expression in the dorsal midbrain is not regulated by Fgf8 and hence remains absent in apc mutants (data not shown). ba, branchial arches; ce, cerebellum; MHB, mid–hindbrain boundary; ot, optic tectum; ov, otic vesicle. Scale bar 125 μm. |
|
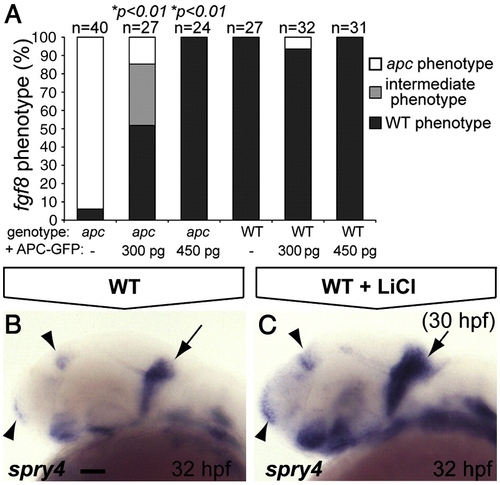
The IsO phenotype as revealed by fgf8 labeling is mainly the result of β-catenin stabilization. (A) Percentage of genotyped mutant, wild-type and heterozygous sibling embryos, showing mutant (white), intermediate (gray) or wild-type (black) phenotypes upon injection of 300 or 450 pg apc-GFP mRNA. The number of injected embryos is indicated above the bars. Injection of 450 pg apc-GFP mRNA completely rescues fgf8 patterning defects. (B, C) LiCl treatments at 30 hpf induces expanded expression at the MHB (arrow), telencephalon and diencephalon (arrowheads) of the Fgf target gene spry4 (C). Lateral view. Anterior to the left. Statistics: two-tailed Fisher′s exact probability test for a table of frequency data. p-values are indicated above the bars. Scale bar 50 μm. EXPRESSION / LABELING:
|
|
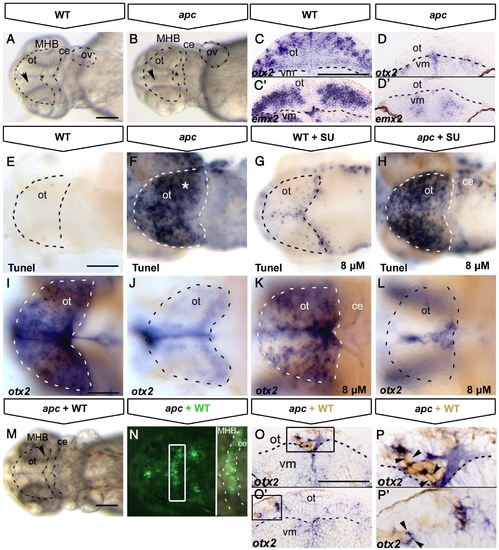
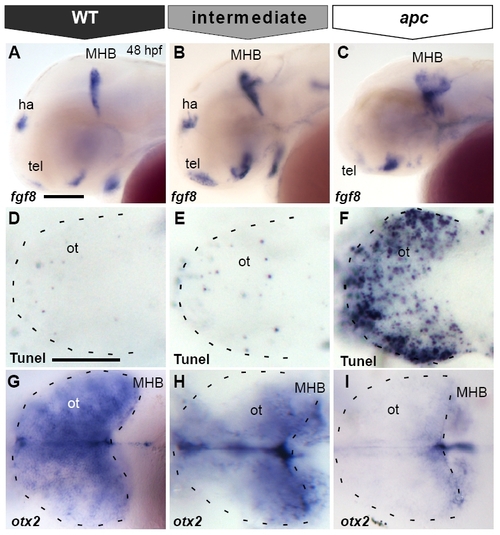
Tectal cell death in apc mutants is not caused by ectopic Fgf8 signalling. All embryos at 48 hpf (A, B, E–N) Dorsal view, anterior to the left. (C–D;O–P) Transversal sections of the midbrain, dorsal up. (A, B) Transmission images showing the collapsed ventricles (asterisk) and the opaque tectum (arrowhead) in apc mutants (B). Dashed lines indicate OT outline, MHB, cerebellum and otic vesicle. otx2 (C, D) and emx2 (C′, D′) expression in optic tectum of apc mutants (D, D′) is abolished. Note the reduction in tectal tissue in mutants. Dashed line indicates dorsal/ventral midbrain boundary. (E–H) Tunel assay shows that blocking ectopic Fgf8 signalling by 8 μM SU5402 treatment (G, H) does not rescue cell death in the mutant tectum (H). (I–L) otx2 expression is mainly unaltered in wild-type (K) and mutant embryos (L) upon SU5402 treatment. Dashed lines indicate OT outline. (M, N) Tectum and MHB morphology of apc embryo at 48 hpf with wild-type cells transplanted into MHB is similar to untransplanted apc embryo (compare to A, B). (N) Overlay with fluorescence to show location of transplanted wild-type cells (green) in MHB (right panel — magnification of boxed area). Dashed lines indicate the MHB and cerebellum. (O–P) Transverse sections of mutant embryos with transplanted WT cells (brown), stained for otx2 (blue). otx2 is restored in wild-type cells transplanted into apc mutant tectum (boxes in panels O, O′ magnified in panels P, P′. Arrowheads indicate single transplanted cells. Dashed line indicates dorsal/ventral midbrain boundary. ce, cerebellum; ot, optic tectum; vm, ventral midbrain. Scale bar 125 μm. |
|
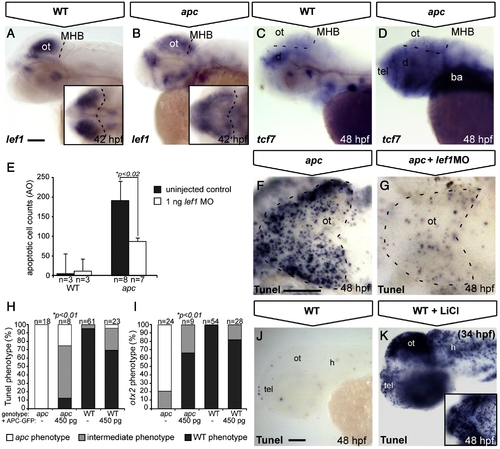
Tectal phenotypes of apc mutants are mainly due to stabilization of β-catenin. (A–D, J, K) Lateral view. (F, G) Dorsal view. Anterior to the left (A, C) In apc mutants (B), lef1 expression is diffusely spread out throughout the dorsal brain, as compared to a strong focal expression in the wild-type OT (A). Insets — dorsal view. Dashed line indicates MHB. (C, D) In wild-types (C), tcf7 is expressed at low levels in the tectum, whereas in apc mutants (n = 12, D), it is upregulated throughout the brain. Dashed lines indicate MHB and dorsal/ventral midbrain boundary. (E–G) Microinjection of 1 ng of a translation-blocking lef1 morpholino (zflef1 MO) into apc mutants partially restores cell survival as shown by quantification of acridine orange (AO)+ cells (E) and Tunel assay (F, G). (H, I) Percentage of genotyped mutant, wild-type and heterozygous sibling embryos, showing mutant (white), intermediate (gray) or wild-type (black) apoptosis (H) and patterning (I) phenotypes upon injection of 450 pg apc-GFP mRNA. Microinjection of 450 pg of apc-GFP mRNA into apc mutants significantly rescues cell survival (H) as well as otx2 expression (I). The number of injected embryos is indicated above the bars. (J, K) Late LiCl treatment of wild-type embryos at 34 hpf (K) phenocopies apoptosis in the apc mutant tectum (F) as observed by Tunel assay. Inset — dorsal view. Statistics: (E) two-tailed Student′s t-test, *p < 0.02; error bars represent SEM; (H, I) two-tailed Fisher′s exact probability test for a table of frequency data, *p < 0.01. ba, branchial arches; d, diencephalon; h, hindbrain; MHB, mid–hindbrain boundary; ot, optic tectum; tel, telencephalon. Scale bar 100 μm. |
|
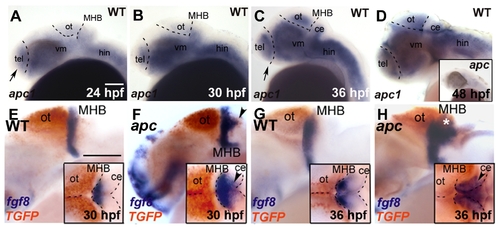
Expression kinetics of apc, fgf8 and TGFP during development of apc mutants. Lateral view. Anterior to the left. (A–D) apc1 expression in wild-type embryos during development. At 24 hpf (A) and 30 hpf (B), apc1 is expressed in posterior telencephalon, cerebellum and ventral mid- and hindbrain. (C) At 36 hpf, apc1 expression expands dorsally into the tectum and anteriorly into the entire telencephalon. (D) At 48 hpf, apc1 is present almost ubiquitously throughout the brain. Inset in D — apc1 expression in apc mutant is strongly reduced, indicating non-sense mediated decay of mRNA. (E–H) Double WISH for fgf8 (blue) and TGFP (red). Insets — dorsal view. Dashed lines indicate the dorsal midbrain midline, MHB and cerebellum. (E, F) At 30 hpf, fgf8 expression in mutants expands into the domain ectopically expressing TGFP (arrowheads in F). (G, H) At 36 hpf, fgf8 partially overlaps with ectopic TGFP in the cerebellum (arrowhead and asterisk in H). ce, cerebellum; hin, hindbrain; MHB, mid/hindbrain boundary; ot, optic tectum; tel, telencephalon; vm, ventral midbrain. Scale bar 125 μm. |
|
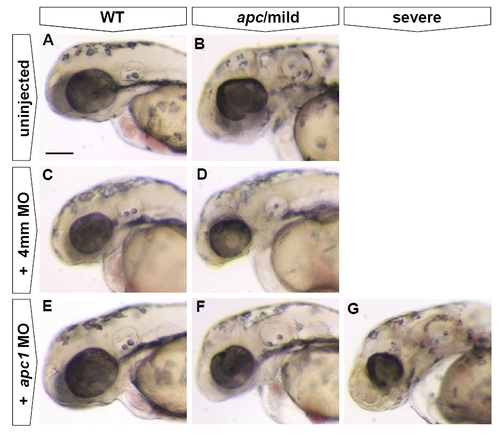
Injection of Apc1, but not control MO, causes exacerbation of the apc mutant phenotype. All embryos at 48 hpf. Lateral view. Anterior to the left. In uninjected embryos, only wild-types (n = 38) and (‘mild’) apc (n = 18) phenotypes were observed. Injection of 0.25 mM apc1 MO into progeny of apc+/- fish causes exacerbation of the apc phenotype (E–G), with 26% of injected embryos (total n = 61) showing no phenotype (E), 56% showing a ‘mild’ phenotype (F) and 18% showing a ‘severe’ phenotype (G). In contrast, injection of 0.25 mM of a 4 mismatch (4mm) control MO into progeny of apc+/- fish did not affect the apc phenotype as only wild-type (C, n = 50) and ‘mild’ apc phenotypes (D, n = 10) were observed. In 4mm-injected wild-type and apc mutants, the size of the eye was somewhat reduced, indicating a non-specific effect of 4mm MO injection. Scale bar 250 μm. |
|
Misexpression of apc-GFP mRNA in apc mutants results in various degrees of phenotypic rescue. (A–C) Lateral view. (D–I) Dorsal view, with eyes removed in (G–I). Anterior to the left. All embryos at 48 hpf. Phenotypes of fgf8 expression (A–C), apoptosis (D–F) and otx2 expression (G–I) as categorized in Figs. 6A and 8H, I. Wild-type or completely rescued apc-GFP injected mutant embryos show normal expression domains of fgf8 at the MHB, habenula (ha) and telencephalon (A), virtually no apoptosis in the tectum (D) and otx2 expression throughout the tectum (G). In partially rescued embryos, fgf8 expression is restored at the habenula and telencephalon, but is still expanded at the MHB (B), similarly to control mutants (C). Partially rescued mutants show a much improved cell survival as compared to the apc phenotype (F). However, there is still a low level of apoptosis in the tectum (E) as compared to the wild-type phenotype (D). otx2 expression is still slightly reduced in partially rescued mutants (H). ha, habenula; MHB, mid–hindbrain boundary; ot, optic tectum; tel, telencephalon. Scale bar 125 μm. |
|
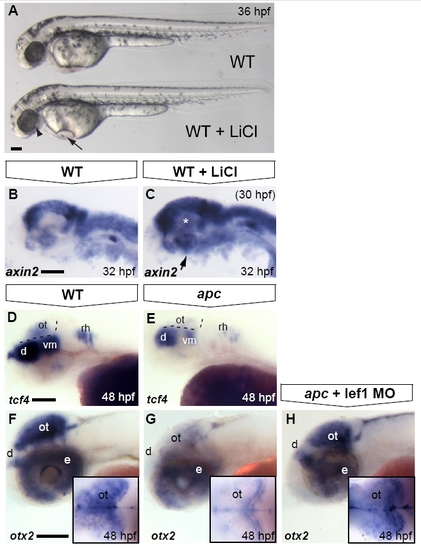
Optic tectum phenotypes in apc mutants are mainly due to overactivated Wnt/β-catenin signalling. Lateral view. Anterior to the left. (A) LiCl treatments at late stages cause morphological defects in the eye (arrowhead), otic vesicles and heart (arrow) similar to the apc phenotype. (B, C) Upon late LiCl treatment at 30 hpf (C), the Wnt/β-catenin target gene axin2 is enhanced in the dorsal brain and induced in the ventral brain (n = 19; asterisk and arrow). (D, E). tcf4 is expressed in the OT of wild-types (D), whereas it is absent from the mutant tectum (n = 12; E) Dashed lines indicate MHB and ventral/dorsal midbrain boundary. (F–H) Loss of otx2 expression in the apc optic tectum is restored to near wild-type expression levels (H) upon Lef1 knockdown using 1 ng of lef1 MO (strong rescue in 50% of apc mutants; remaining mutants show weak induction; n = 23). Insets — dorsal view with eyes removed. d, diencephalon; e, eye; ot, optic tectum; rh, rhombomere; vm, ventral midbrain. Scale bar 125 μm. |
|
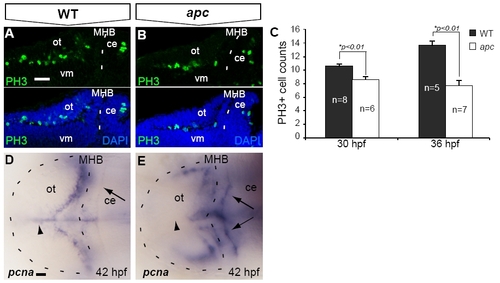
Reduced proliferation in the tectum opticum of apc mutants. (A–C) At 30 hpf, confocal analysis identified fewer PH3+ mitotic cells in the apc mutant OT. (B) as compared to wild-types (A). (C) The reduction of PH3+ cell numbers was augmented during further development. (D, E) Reduction of proliferation in the mutant OT is corroborated by the absence of pcna labeling from the mutant OT midline (arrowhead; n = 5). Of note is enhanced ectopic expression of pcna in the mutant cerebellum (arrows). Statistics: two-tailed Student′s t-test, *p < 0.01. Error bars represent SEM. ce, cerebellum; MHB, mid–hindbrain boundary; ot, optic tectum; vm, ventral midbrain. Scale bar 100 μm. |
Reprinted from Developmental Biology, 331(2), Paridaen, J.T., Danesin, C., Elas, A.T., van de Water, S., Houart, C., and Zivkovic, D., Apc1 is required for maintenance of local brain organizers and dorsal midbrain survival, 101-112, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.