- Title
-
dazed gene is necessary for late cell type development and retinal cell maintenance in the zebrafish retina
- Authors
- Perkins, B.D., Nicholas, C.S., Baye, L.M., Link, B.A., and Dowling, J.E.
- Source
- Full text @ Dev. Dyn.
|
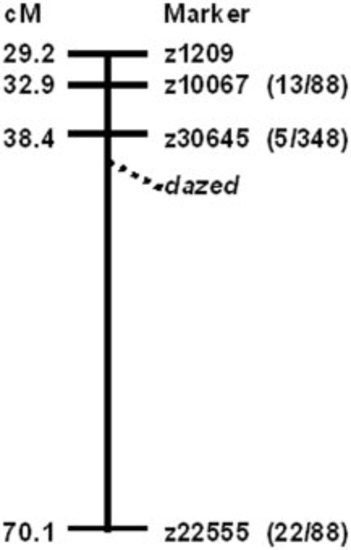
Genetic mapping revealed dazed is on linkage group 3 (LG3). A schematic cartoon of LG3 shows the genetic position of four SSLP z-markers and the approximate position of the dazed locus. The numbers in parentheses represent the number of recombinants and the total number of mutants tested for each SSLP marker. |
|
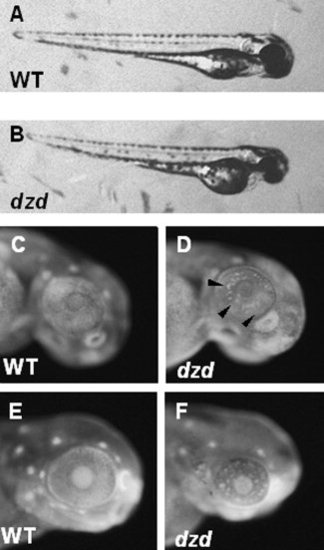
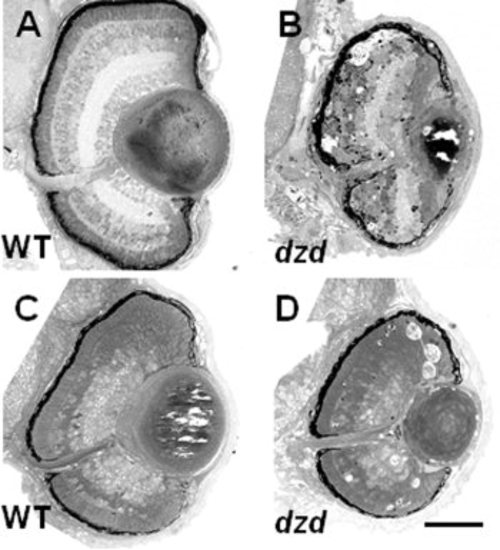
Morphological, histological, and cell death characterization of the dazed mutant. A,B: Lateral view of wild-type (WT, A) and dazed (B) embryos at 3 days postfertilization. C-F: Acridine orange staining on PTU-treated embryos at 48 hours postfertilization (hpf, C,D) and 72 hpf (E,F) reveals cell death in dazed embryos. Dying cells are easily observed at 48 hpf in dazed embryos (D, arrowheads) and at 72 hpf. |
|
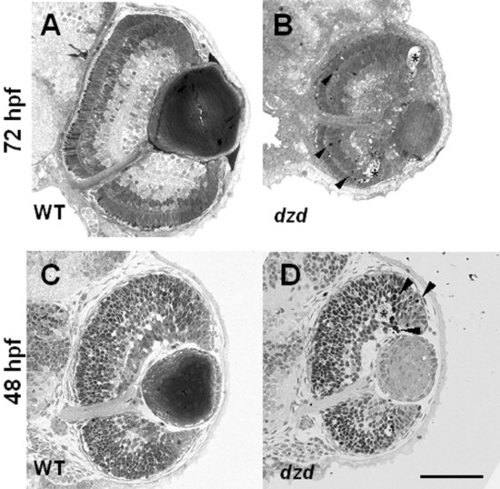
A-D: Transverse sections on wild-type (A,C) and dazed (B,D) embryos was done at 72 hours postfertilization (hpf) and 48 hpf. Darkly staining pyknotic cells (arrowheads in B,D) were observed near the outer nuclear layer at 72 hpf and at the border of the marginal zone at 48 hpf in dazed embryos. Acellular holes were observed at both 72 hpf and 48 hpf in dazed embryos (asterisks). Scale bar = 80 μm in D (applies to A-D). |
|
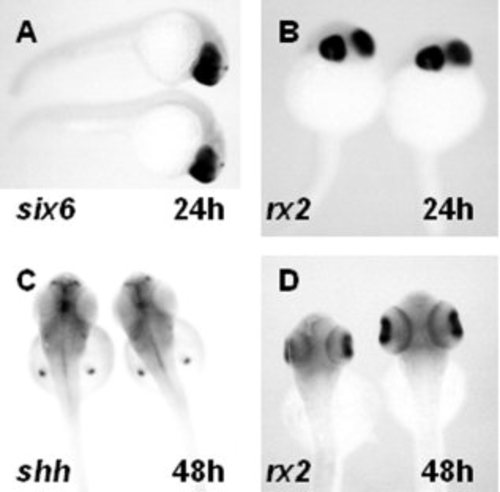
Gene expression analysis for genes involved in optic cup formation and outer nuclear layer differentiation. A,B: In situ hybridization with mRNA probes of six6 (A) and rx2 (B) at 24 hours postfertilization (hpf). C,D: Dorsal views of embryos from clutches of dazed heterozygous matings were analyzed for the expression of (C) shh or (D) rx2 at 48 hpf. Embryos (n ≥ 20) from clutches of dazed heterozygous matings showed no difference in expression patterns of any genes tested. |
|
BrdU labels proliferating cells in wild-type and dazed retinas. A-C: BrdU incorporation during a 24-hr pulse from 72 to 96 hours postfertilization (hpf) in wild-type (WT, A) and dazed mutants (B,C). Proliferating cells are seen in both the dorsal and ventral marginal zones of wild-type animals (arrows). Far fewer cells are seen in most dazed mutants (B) and some mutants have little to no labeling (asterisk in C). Scale bar = 80 μm in C (applies to A-C). |
|
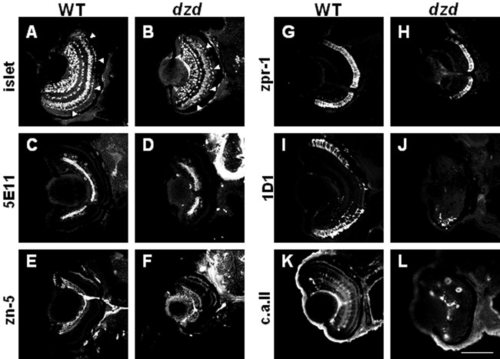
Retinal cell type marker analysis of dazed mutants at 72 hours postfertilization (hpf). Central, transverse retinal sections of wild-type (WT, left) and dazed (right) were labeled with cell type-specific antibodies and visualized with appropriate secondary antibodies (see text for details). A,B: Islet-1 immunoreactivity; ganglion, bipolar, and horizontal cells and their precursors. C,D: 5E11 antigen; amacrine cells and their processes. E,F: zn-5 antigen; ganglion cells and their processes. G,H: zpr-1/fret-43 antigen; red-green double cones. I,J: 1D1 antigen; rod photoreceptors. K,L: Carbonic anhydrase II (c.a. ii) immunoreactivity; Müller glial cells and nonocular ectoderm. Scale bar = 80 μm in L (applies to A-L). EXPRESSION / LABELING:
|
|
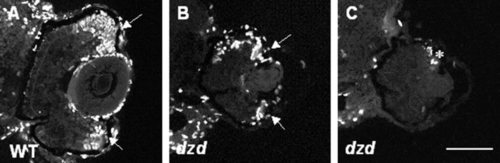
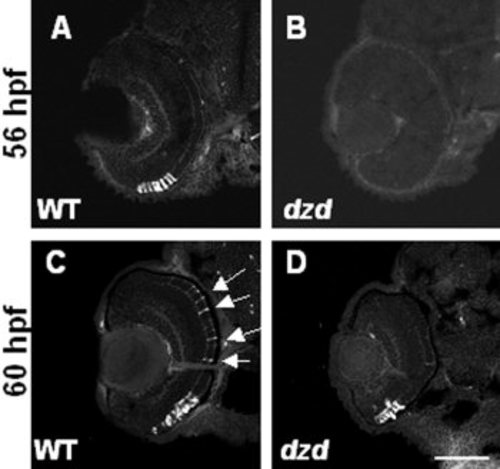
Marker analysis of early rod photoreceptor appearance. A-D: 1D1 antibodies were used to label rod photoreceptor cells in wild-type (WT, left) and dazed (right) embryos at either 56 hours postfertilization (hpf, A,B) or 60 hpf (C,D). Note that rods are present in the central and dorsal retina in wild-type embryos at 60 hpf (arrows) but not dazed embryos. Also notice the similarity in staining between wild-type at 56 hpf and dazed at 60 hpf (compare A with D). Scale bar = 90 μm in D (applies to A-D). |
|
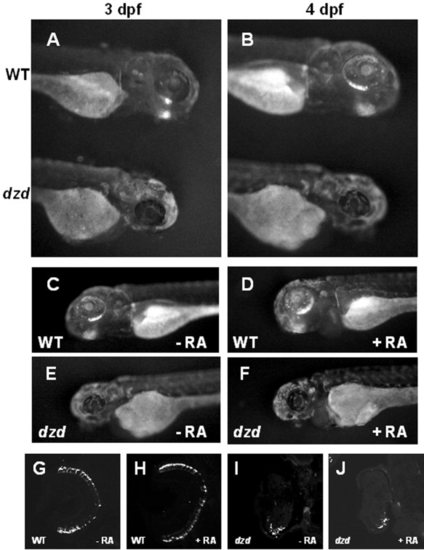
Analysis of the dazed mutation in a transgenic line and after retinoic acid treatments. The dazed mutation was bred into a transgenic line (Perkins et al., [2002]) expressing a GFP-CT44 fusion protein under the control of the opsin promoter to visualize rod photoreceptors. A,B: At 3 and 4 days postfertilization (dpf), green fluorescent protein (GFP) was observed in the ventral retinas of wild-type (WT, top) but not dazed (bottom) embryos. C-F: Wild-type embryos treated from 48-96 hours postfertilization (hpf) with 300 nM retinoic acid (48-hr treatment) had more GFP expression, whereas retinoic acid had no effect on GFP expression in dazed mutants. G-J: Central, transverse sections from retinoic acid treated and control embryos were stained with 1D1 and confirmed that rods were more numerous in retinoic acid treated wild-type animals (H) than control (G) but no increase was observed in dazed mutants (I,J). |
|
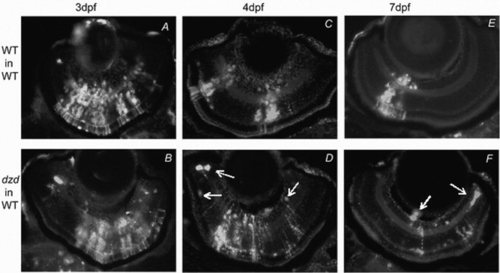
Mosaic analysis of retinal survival and differentiation for the dazed mutation. Mosaic retinas were produced by blastomere transplantation and donor cells were labeled by fluorescence (white cells). A-F: Transplanted wild-type or dazed cells were allowed to develop in wild-type hosts for 3 days (WT, A,B), 4 days (C,D), or 7 days (E,F). For the dazed donor cells, cell death was observed beginning on day 4 and continuing through day 7 as indicated by pyknotic cells, cellular debris (D,F arrows), and a decrease in clone size. These observations indicate that the dazed mutation is cell-autonomous for cell survival. Experimental groups: A, n = 9; B, n = 5; C, n = 13; D, n = 14; E, n = 4; F, n = 6. |
|
Constant light rearing exacerbates cell death in dazed mutants. A: Wild-type (WT) retinas did not exhibit defects after constant light treatment. B: Retinas from dazed mutants raised under constant lighting displayed widespread cell death in all cell layers and large holes in the photoreceptor layer both centrally and peripherally. C: Wild-type retinas from animals raised in constant darkness appeared slightly developmentally delayed, but degeneration was not observed. D: Dark-reared dazed retinas degenerate. Pyknotic cells were found in the photoreceptor layer, and acellular holes were seen at the marginal zones as in animals raised under cyclic light. Scale bar = 70 μm in D (applies to A-D). |
|
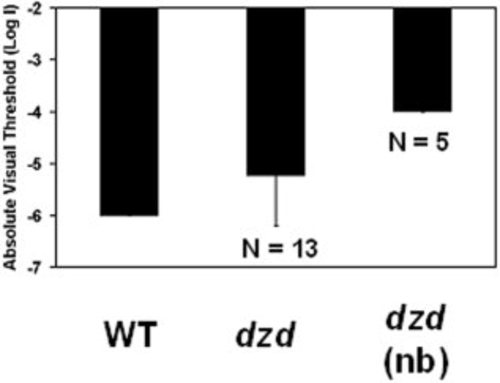
Analysis of visual thresholds by the escape response behavioral test. Wild-type (WT) adults (minimum 12 months of age; n = 10) have a visual threshold of 6 log units below unattenuated light (log I = -6), which is consistent with published results (Li and Dowling, [1997]). Visual thresholds of heterozygous dazed siblings were elevated at least 0.8 log units to an average absolute threshold of log I -5.2 with a large variation (SD = 0.9) when tested as a population (n = 13). Visual thresholds were elevated by at least 2 log units (log I > -4.0) in heterozygous fish that were identified as night blin |