- Title
-
Investigation of the role of VHL-HIF signaling in DNA repair and apoptosis in zebrafish
- Authors
- Kim, H.R., Santhakumar, K., Markham, E., Baldera, D., Greenald, D., Bryant, H.E., El-Khamisy, S.F., van Eeden, F.J.
- Source
- Full text @ Oncotarget
|
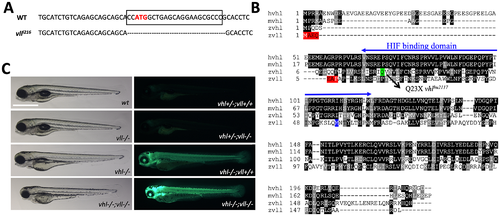
Figure 1: A vll mutant allele, vll216, is generated by a zinc finger nuclease. (A) The mutant allele harbors 23 nucleotide deletion around translational starting site. (B) Zebrafish Vll shares 52% homology with human VHL. The seven amino acids that are lost in vll216 are highlighted in red. Even if the next available ATG (highlighted in blue) were to be used as an alternative translational start site, it would lead to a protein that lacks most of the predicted Hif binding domain. (C) Since phd3 (egln3) expression most dramatically reflects the increased Hif expression, we used Tg (phd3:: EGFP)i144 transgenic line as a Hif signaling readout. Zebrafish vll–/– embryos do not show any morphological phenotype and the Tg (phd3:: EGFP)i144 expression in the mutants was identical to that in the wild type embryos. Reflecting the role of Vll in Hif regulation in the absence of Vhl, there was an enhanced GFP expression in the vhl–/–; vll–/– double mutant embryos in comparison to that in vhl–/– embryos. Scale bar: 1 mm. PHENOTYPE:
|
|
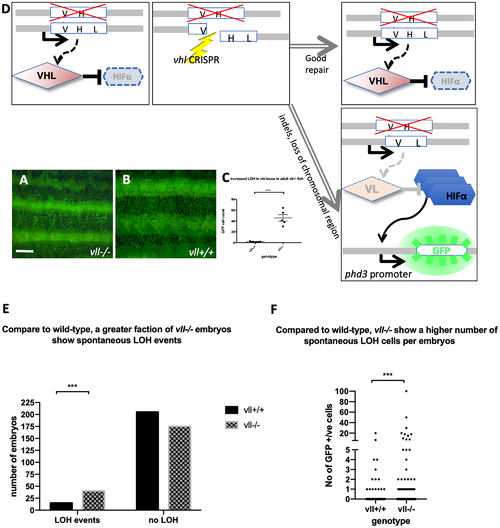
The adult vll–/– mutant fish are more susceptible to LOH in the vhl locus. (A and B) When we examined 6 months old vll–/– mutant fish, there was an increased number of spontaneous LOH in the vhl locus in vll–/– mutant fish in comparison to vll+/+ wild type fish. The increased LOH in vll–/– mutant fish was quantified in (C) ****p < 0.0001, unpaired t-test. Scale bar: 1 mm (D) Schematic diagram illustrating the principle of our reporter system that if DNA damage is introduced into the remaining wildtype vhl allele, for instance by a CRISPR, in the vhl+/–: Tg (phd3:: EGFP)i144 fish in response to the genotoxic stress and the damage is well repaired and no frameshift is introduced, the cells will remain EGFP negative. However, if the DNA damage introduced into the wild type allele is not repaired properly, the cells will lose heterozygosity and express very bright EGFP expression. (E) we also examined the spontaneous LOH at the vhl locus in vll–/– mutant embryos. Although the number of LOH events was much lower than that in adults and a majority of embryos did not have any LOH events, we found a statistically significant increase in the number of embryos that experienced a LOH event in vll–/– embryos in comparison to wild type embryos. ****p < 0.0001, Chi-square (and Fisher’s exact) test. (F) There was an increase in the number of EGFP positive cells in vll–/– embryos in comparison to wild type embryos. ***p < 0.001, unpaired t-test. PHENOTYPE:
|
|
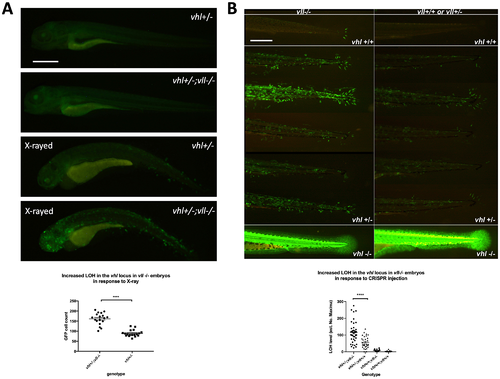
There is an increase in LOH in vll–/– embryos in response to X-ray treatments and CRISPR injection. (A) There was an increased LOH in the vhl locus in vll–/– embryos in response to X-ray treatments. ****p < 0.0001, unpaired t-test. (B) Injection of gRNA/Cas9 against vhl locus induced increased LOH at the vhl locus in vll–/– embryos in comparison to vll+/+ wild type embryos. B is a composite image of 14 individual tail images, white lines separate different genotypes. ***p < 0.001, one way ANOVA; Holm-Sidak’s multiple comparison test. Scale bars: 0.5 mm. PHENOTYPE:
|
|
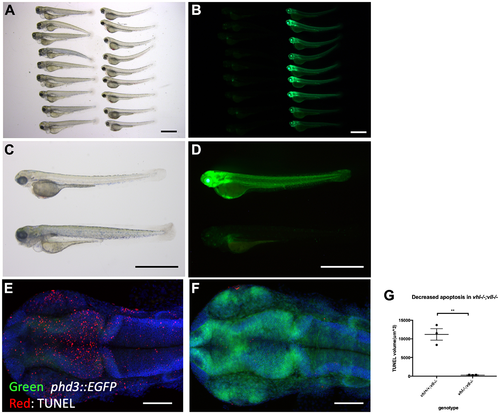
vhl–/–; vll–/– double mutants are protected from X-ray induced apoptosis. (A–D) The embryos from vhl+/–; vll–/– pair mating were collected and the embryos were exposed to X-ray treatment at 1dpf. Then the treated embryos were examined at 5dpf. There were clear differences in response to X-ray treatment; majority of embryos were severely affected by X-ray treatment whereas around 25% of the embryos were well protected without much sign of cell death. We classified these embryos and looked at them under the fluorescent microscope. This revealed that all the protected embryos were EGFP positive vhl–/–; vll–/– double mutants. (E and F) The embryos were collected from the vhl+/–; vll–/– pair mating, and then exposed to X-ray at 1dpf. The embryos were fixed 3 hours post X-ray and we analysed the cell death in the head region with TUNEL. This showed extremely decreased TUNEL positive cells in the vhl–/–; vll–/– double mutants in comparison to their siblings. (G) Quantification of TUNEL stain. n = 3, **p < 0.01, unpaired t-test. Scale bars: 1 mm (A–D) and 0.2 mm (E&F). PHENOTYPE:
|
|
( PHENOTYPE:
|
|
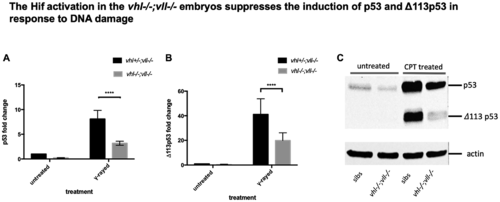
p53 expression in the vhl–/–; vll–/– double mutants is downregulated in comparison to that in their siblings. (A and B) Embryos from vhl+/–; vll–/– pair mating were collected and irradiated at 2dpf with γ-ray at a dose of 20 gray. q-PCR was performed 24 hours post γ-ray to quantify the full length of p53 and short form of Δ113p53. This revealed that there was a significant decrease in both forms of p53 expression in the vhl–/–; vll–/– embryos in comparison to their siblings. ****p < 0.0001, two way ANOVA test. The statistical analysis was performed with ddCt values. (C) We also treated embryos from vhl+/–; vll–/– incross with 10 nM CPT and both isoforms of p53 induction was examined in the vhl–/–; vll–/– embryos and in their siblings by Western blot. After treatment, there was a clear reduction in both isoforms of p53 expression in the vhl–/–; vll–/– embryos in comparison to their siblings. |
|
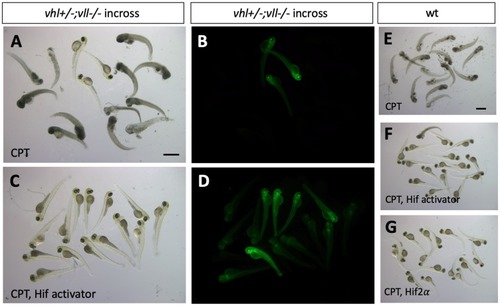
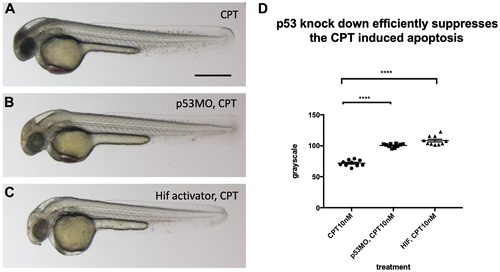
p53 knock down by p53 morpholino injection protects embryos from CPT induced apoptosis. (A) Embryos collected from vhl+/–; vll–/– pair mating were treated with 10 nM CPT. This resulted in severe cell death in the CPT treated embryos with the exception of GFP positive vhl–/–; vll–/– double mutants. (B) On the contrary, when p53 morpholino was injected into the embryos prior to 10 nM CPT treatment, all the embryos were protected from CPT induced apoptosis. (C) The similar protection was also observed in the embryos treated with Hif activator prior to CPT treatment. Only GFP negative embryos were imaged and quantified for this experiment. (D) The image quantification of levels of apoptosis using gray scale in ImageJ, low gray scale values denote high levels of apoptosis. ****p < 0.0001, one-way ANOVA. Scale bar: 0.5 mm. |
|
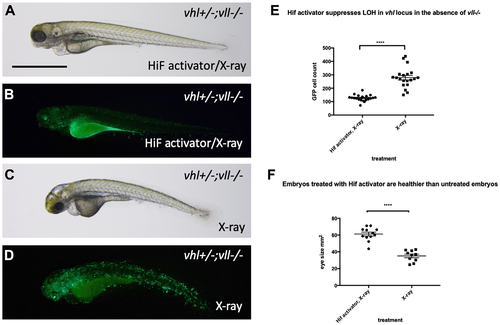
Hif activator treatment protects embryos from X-ray induced DNA damage. (A–D) We investigated the effect of Hif activation on the increased LOH in vhl locus in vll–/– mutant embryos. When embryos were treated with Hif activator prior to X-ray treatment, the increased LOH in the vhl locus in vll–/– embryos was significantly reduced (B) in comparison to vll–/– embryos that were exposed to X-ray without Hif activator treatment (D). (E) The effect of Hif activator on the LOH suppression in the vhl locus was quantified. ****p < 0.0001, unpaired t-test. Hif activator treated vll–/– embryos also looked more healthier (A) than untreated embryos (C). (F) The size of eyes was measured as a surrogate for general health, and quantified in the Hif activator treated and untreated embryos. ****p < 0.0001, unpaired t-test. Scale bar: 1 mm. |
|
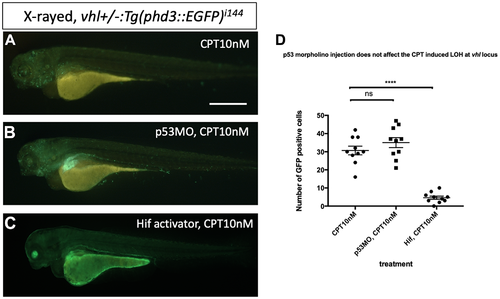
p53 knock down by p53 morpholino does not affect the number of LOH at the vhl locus induced by CPT. (A) The embryos collected from vhl+/–; vll–/– pair mating were treated with 10 nM CPT. There was GFP positive cells throughout the CPT treated embryos indicating the LOH in these cells. (B) A similar number of GFP positive cells were observed in the p53 morpholino injected embryos suggesting that the CPT induced LOH formation in these embryos is independent of p53 level. (C) On the contrary, the treatment of Hif activator prior to CPT treatment abolished the LOH formation in the embryos, signifying the genoprotective role of Hif against CPT treatment. (D) The number of EGFP positive cells were quantified using ImageJ (n = 10 per treatment). nsp > 0.05, ****p < 0.0001, one-way ANOVA. Scale bar: 0.5 mm. |
|
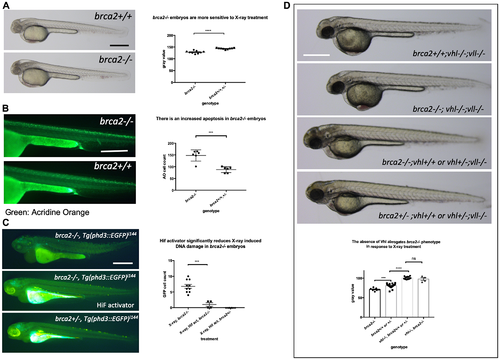
The elevated Hif level in the vhl–/– mutants alleviates cell deaths induced by the mutation in brca2. (A) brca2–/– embryos were more sensitive to X-ray treatment in comparison to their siblings demonstrating the increased cell death in CNS. ****p < 0.0001, unpaired t-test. (B) The Acridine Orange staining confirmed the increased apoptosis in the brca2–/– embryos. ***p < 0.001, unpaired t-test. (C) X-ray treatment induced a few EGFP positive cells in the brca2–/– embryos, indicating the loss of both vhl alleles in these embryos. On the other hand the EGFP positive cells were hardly observed in their siblings. The Hif activator treatment suppressed the appearance of EGFP positive vhl mutant cells in the brca2–/– embryos. ***p < 0.001, one way AVOVA. (D) brca2–/– embryos were more sensitive to X-ray treatment in comparison to their siblings. However, when the vhl –/–;vll –/– mutations are introduced in the brca2–/– embryos, increased apoptosis in the CNS of brca2–/– embryos was suppressed and the brca2–/– embryos were indistinguishable from their siblings. ***p < 0.001, ****p < 0.0001, nsp > 0.05, one way ANOVA. Scale bars: 0.5 mm. PHENOTYPE:
|
|
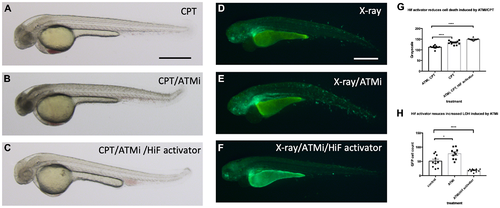
Hif activation abolishes the sensitivity to CPT induced cell death in ATM inhibitor treated embryos. (A) The wild type embryos were treated with 10 nM CPT to induce cell death in the CNS. (B) When the embryos were treated with ATMi before CPT treatment, the embryos were highly sensitised to CPT treatment and induced severe cell death in the CNS. (C) When the embryos were treated with Hif activator in combination with ATMi prior to CPT treatment, the embryos were well protected from CPT/ATMi induced cell death in CNS. The quantification of the data is shown in (G) ****p < 0.0001, one way ANOVA. (D) The vhl+/–; vll–/–; Tg (phd3:: EGFP)i144 embryos were treated with X-ray to induce LOH at the vhl locus. (E) When the vhl+/–; vll–/–; Tg (phd3:: EGFP)i144 embryos were treated with ATMi before the X-ray treatment, there was a dramatic increase in the LOH in the vhl locus in the ATMi treated embryos. (F) When the embryos were treated with Hif activator in combination with ATMi before the X-ray treatment, there was a remarkable reduction in the LOH in the vhl locus. The quantification of the data is shown in (H) *p < 0.05, ****p < 0.0001, one way ANOVA. Scale bars: 0.5 mm. |
|
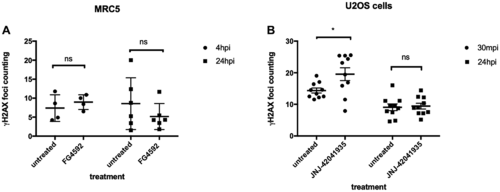
HIF activation does not protect mammalian cells from X-ray induced DNA damage. (A) We treated MRC5 cells with HIF activator FG 4592 for 3 hours prior to X-ray treatment at 5 gray. The cells were fixed at 4 hpi and 24 hpi with 4% PFA and stained with γH2AX. This revealed that there was no significant difference in the γH2AX formation between HIF activator treated cells and non-treated cells. nsp > 0.05, one way ANOVA. (B) The cells were treated with HIF activator JNJ-42041935 for 3 hours prior to X-ray treatment at 2 gray. The cells were fixed at 30 mpi and 24 hpi and stained with γH2AX. At both time points, we could not observe the reduction in γH2AX formation in the HIF activator treated group in comparison to untreated group. At 30 mpi, there was even an increase in the number of γH2AX foci in the HIF activator treated group in opposition to our expectation. nsp > 0.05, *p < 0.05, one way ANOVA. |
|
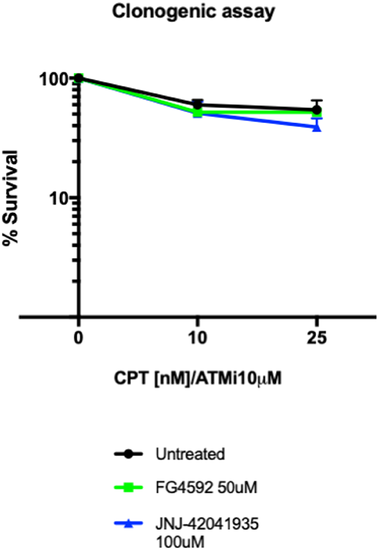
Hif activator treatment does not affect the survival of MRC5 cells. 4000 MRC5 cells were seeded in 6 well plates and left to grow overnight. The next day, the cells were treated with HIF activator (FG4592 or JNJ-42041935) and ATMi for 3 hours. CPT was added at either 10 nM or 25 nM concentration for 1 hour and the drugs were washed off. The colony formation was examined 7 days after the drug treatment. CPT/ATMi treatment decreased the survival rates but we could not find any effect of HIF activator on the survival of MRC5 cells. nsp > 0.05, unpaired t-test. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|