- Title
-
Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression
- Authors
- Gurevich, D.B., Severn, C.E., Twomey, C., Greenhough, A., Cash, J., Toye, A.M., Mellor, H., Martin, P.
- Source
- Full text @ EMBO J.
|
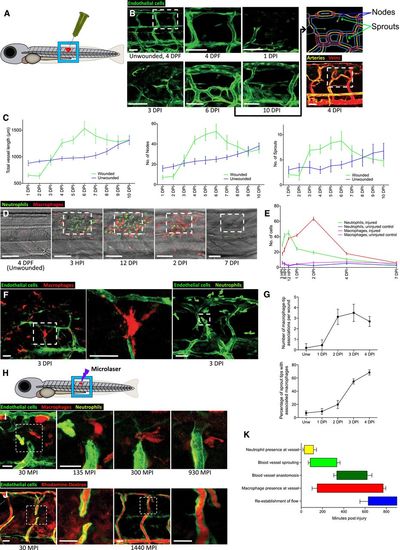
Macrophages are associated with blood vessel sprouting tips in zebrafish wounds
|
|
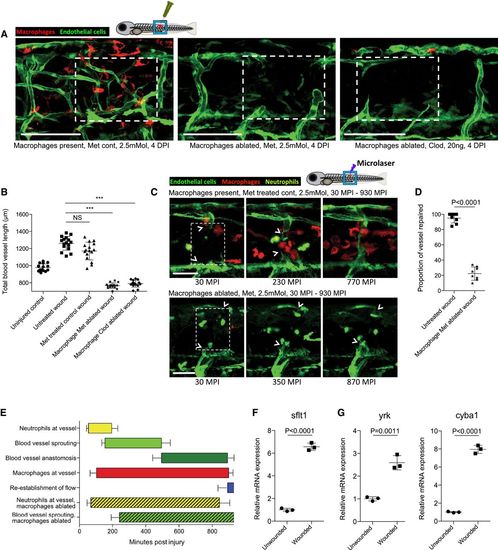
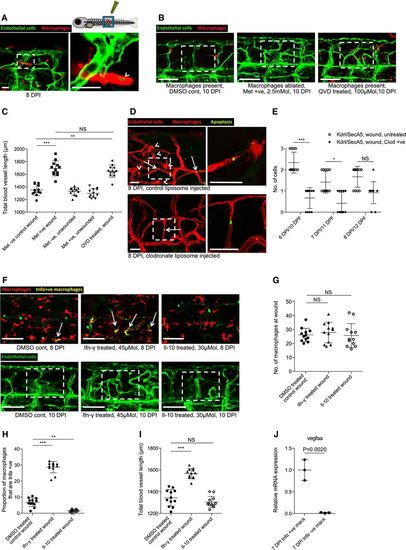
Macrophages are necessary for wound angiogenesis and act to stabilise wound blood vessel sprouting tips Representative confocal projection images of needle‐stick injured Tg(fli:GFP); Tg(mpeg:mCherry) or Tg(fli:GFP); Tg(mpeg:KalTA4); Tg(UAS:nfsB‐mCherry) zebrafish in combination with various treatments (as indicated), for ablating macrophages (red) during the peak stage of vessel (green) sprouting (4 DPI). Boxed area denotes wound site. In control (DMSO vehicle) and metronidazole‐treated fish lacking nitroreductase expressing macrophages, a normal inflammatory response and wound angiogenesis is observed. Upon ablation of macrophages using the nitroreductase–metronidazole system, wound angiogenesis is almost completely blocked. The same is true when macrophages are ablated using clodronate liposome injections. Quantification of total blood vessel length in the presence/absence of macrophages at the wound site, measured from images represented in (A), using Angioanalyser. N = 16 independent fish per condition. Statistical significance, as determined by one‐way ANOVA, is P ≤ 0.0001. Subsequent Bonferroni multiple comparison test, determines level of significance, as indicated. Significance values: ***P ≤ 0.0001. Images taken from timelapse movies of 4 DPF laser wounded Tg(fli:GFP); Tg(mpx:GFP); Tg(mpeg:mCherry) control or Tg(fli:GFP); Tg(mpx:GFP); Tg(mpeg:KalTA4); Tg(UAS:nfsB‐mCherry) macrophage ablation transgenic zebrafish, treated with metronidazole and imaged 30–930 min post‐injury (MPI). Boxed area denotes wound site. Arrowheads indicate position of damaged, sprouting vessel tips. Quantification of proportion of vessel repaired within 930 MPI, measured from movies represented in (C). N = 8 independent fish per condition. Statistical significance is indicated, as determined by two‐tailed t‐test. Graphical representation of typical cellular interaction events and their time course, measured from movies represented in (C). N = 8 independent fish per condition. Gene expression of FAC‐sorted neutrophils extracted from Tg(mpx:GFP); Tg(mpeg:mCherry) needle‐stick wounded fish at 12 HPI, showing relative gene expression for sflt1 compared to unwounded fish at 4.5 DPF. N = 50 independent fish per condition per replicate, 3 replicates. Statistical significance is indicated, as determined by two‐tailed t‐test. Gene expression of FAC‐sorted macrophages from Tg(mpx:GFP); Tg(mpeg:mCherry) transgenic zebrafish at 12 HPI post‐needle‐stick injury, showing relative gene expression for cyba1 and yrk compared to unwounded fish at 4.5 DPF. N = 50 independent fish per condition per replicate, 3 replicates. Statistical significance is indicated, as determined by two‐tailed t‐test. |
|
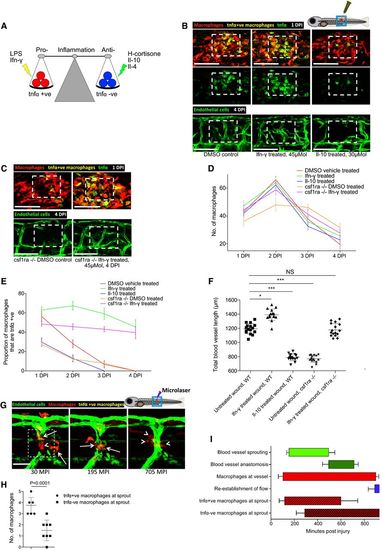
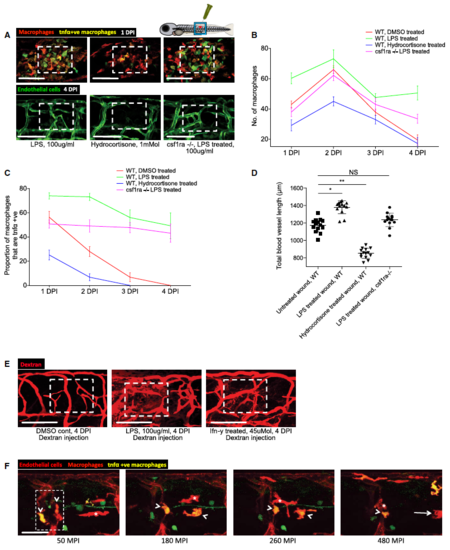
Manipulation of wound inflammation and macrophage phenotypic state affects the extent of wound angiogenesis Schematic showing the factors used to skew macrophage phenotype towards either pro‐inflammatory or anti‐inflammatory states. Representative confocal projection images of needle‐stick injured zebrafish, taken from either 1 DPI Tg(mpeg:mCherry); Tg(tnfα:GFP) transgenic zebrafish (top panel) or 4 DPI Tg(fli:GFP) transgenic zebrafish (bottom panel), treated, as indicated, with DMSO (control), Ifn‐γ or Il‐10. Boxed area denotes wound site. Representative confocal projection images of needle‐stick injured zebrafish taken from either 1 DPI Tg(mpeg:mCherry); Tg(tnfα:GFP) transgenic fish (top panel; middle panel for GFP only) or 4 DPI Tg(fli:GFP) transgenic fish (bottom panel), csf1ra−/− mutant and treated with Ifn‐γ. Boxed area denotes wound site. Quantification of number of macrophages at the wound site between 1 and 4 DPI, measured from images represented in (B and C). N = 14 (from B) and N = 16 (from C) independent fish per timepoint per condition. Quantification of proportion of macrophages that are tnfα positive at the wound site from 1 to 4 DPI, measured from images represented in (B and C). N = 14 (from B) and N = 16 (from C) independent fish per timepoint per condition. Quantification of total blood vessel length at 4 DPI, measured from images represented in (B and C), using Angioanalyser. N = 14 (from B) and N = 16 (from C) independent fish per timepoint per condition. Statistical significance, as determined by one‐way ANOVA, is P ≤ 0.0001. Subsequent Bonferonni multiple comparison test, determines level of significance, as indicated. Significance values: *P ≤ 0.05, ***P ≤ 0.0001. Representative confocal projection images taken from laser wounded Tg(fli:GFP); Tg(mpeg:mCherry); Tg(tnfα:GFP) triple transgenic zebrafish, imaged 30‐930 MPI. Boxed area denotes wound site. tnfα‐positive, inflammatory macrophages (arrows) associate with damaged, sprouting blood vessels earlier and in larger numbers than tnfα‐negative, non‐inflammatory macrophages (arrowheads). Quantification of numbers of inflammatory versus non‐inflammatory macrophages associated with damaged, sprouting blood vessels, measured from images represented in (G). N = 8 independent fish. Statistical significance is indicated, as determined by two‐tailed t‐test. Graphical representation of common cellular interaction events and their time course, measured from movies represented in (G) and compared to events observed in Fig 3C. N = 8 independent fish. |
|
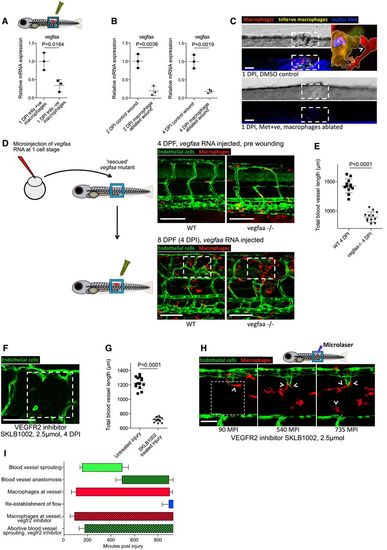
Zebrafish wound angiogenesis is driven by wound inflammatory macrophage‐mediated vegf signalling Gene expression for FAC‐sorted zebrafish wound macrophages from Tg(tnfα:GFP); Tg(mpeg:mCherry) zebrafish, extracted at 1 DPI following needle‐stick injury. tnfα‐positive inflammatory macrophages express higher levels of vegfaa than tnfα‐negative non‐inflammatory macrophages. N = 50 independent fish per condition, 3 replicates. Statistical significance is indicated, as determined by two‐tailed t‐test. Gene expression for whole‐dissected zebrafish needle‐stick wounds from Tg(mpeg:KalTA4); Tg(UAS:nfsB‐mCherry) zebrafish following treatment with metronidazole, versus control DMSO‐treated wounded fish, at 2 DPI and 4 DPI. Macrophage absence from wounds results in an overall decrease in vegfaa levels at the wound site. N = 30 independent fish per condition, three replicates. Statistical significance is indicated, as determined by two‐tailed t‐test. Representative confocal projection images taken from needle‐stick wounded Tg(mpeg:KalTA4); Tg(UAS:nfsB‐mCherry); Tg(tnfα:GFP) triple transgenic zebrafish, DMSO control or metronidazole treated indicated, upon which vegfaa WISH was performed. Inset image shows high levels of vegfaa expression (blue) in tnfα‐positive macrophages (yellow, asterisk) versus low levels in tnfα‐negative macrophages (red, arrowhead). Boxed area denotes wound site. N = 12 independent fish per condition. Schematic and representative confocal images showing rescue experiment and subsequent needle‐stick injury performed on vegfaa homozygous mutant zebrafish or siblings, transgenic for Tg(etv2:GFP). Embryos derived from an incross of vegfaa+/− adults were injected with “rescue” mRNA encoding for vegfaa‐165 at the one‐cell stage. At 4 DPF, these mutant fish had an impaired but functional complement of ISVs. Needle‐stick injuries performed on these mutants and their siblings at 4 DPF indicated a failure of wound angiogenesis by 4 DPI, despite a relatively normal inflammatory response. Boxed area denotes wound site. Quantification of total blood vessel length at 4 DPI, measured from images represented in (D), using Angioanalyser. N = 12 independent fish per condition. Statistical significance is indicated, as determined by two‐tailed t‐test. Representative confocal projection image taken from needle‐stick wounded Tg(fli:GFP) transgenic zebrafish, imaged 4 DPI and treated with vegfr2 inhibitor SKLB1002 from the moment of injury. Boxed area denotes wound site. Quantification of total blood vessel length, measured from images represented in (F) using Angioanalyser. N = 14 independent fish. Statistical significance is indicated, as determined by two‐tailed t‐test. Representative confocal projection images taken from laser wounded Tg(fli:GFP), Tg(mpeg:mCherry) double‐transgenic zebrafish, imaged 30–930 MPI and treated with SKLB1002 from moment of injury. Macrophages still associate with damaged vessels (arrowheads), but these vessels do not sprout appropriately and fail to repair. Boxed area denotes wound site. Graphical representation of common cellular interaction events and their time course, measured from movies represented in (H) and compared to events observed in Fig 3C. N = 8 independent fish. |
|
Macrophages mediate blood vessel regression, dependent on phenotypic status Representative confocal projection images taken from needle‐stick wounded Tg(fli:GFP); Tg(mpeg:mCherry), double‐transgenic zebrafish, imaged at 7 DPI. Some macrophages at this later wound repair timepoint contain phagocytosed GFP tagged endothelial material (arrowhead). Boxed area denotes macrophage containing endothelial cell material. N = 9 independent fish. Representative confocal projection images taken from needle‐stick wounded Tg(fli:GFP); Tg(mpeg:KalTA4); Tg(UAS:nfsB‐mCherry) triple transgenic zebrafish, imaged at 10 DPI, treated with vehicle control, metronidazole or QVD pan‐caspase inhibitor from 6 DPI. Both macrophage ablation and caspase blockade resulted in a failure to remodel vessels. Boxed area denotes wound site. Quantification of total blood vessel length, measured from images represented in (B). N = 12 independent fish per timepoint per condition. Statistical significance, as determined by one‐way ANOVA, is P ≤ 0.0001. Representative confocal projection images taken from needle‐stick wounded Tg(kdrl:mCherry‐CAAX); Tg(secA5‐YFP); Tg(mpeg:mCherry) transgenic zebrafish, imaged at 8 DPI, injected with control liposomes or clodronate liposomes as indicated. Macrophages are observed within the wound site in control liposome‐treated fish (arrowheads). The apoptotic marker is seen in thin, unicellular tubes during the regression phase (arrows). Ablation of macrophages during the regression phase results in decreased levels of vessel apoptosis. Quantification of apoptotic endothelial cells during vessel regression, measured from images represented in (D). N = 12 independent fish per timepoint per condition. Statistical significance, as determined by one‐way ANOVA, is P ≤ 0.0001. Representative confocal projection images taken from either 8 DPI Tg(mpeg:mCherry); Tg(tnfα:GFP) transgenic fish or 10 DPI Tg(fli:GFP) transgenic fish, treated as indicated from 6 DPI, following needle‐stick injury. Inflammatory tnfα‐positive macrophages are indicated (arrows). Boxed area denotes wound site. Quantification of number of macrophages at the wound site at 8 DPI, measured from images represented in (F). N = 12 independent fish per timepoint per condition. Statistical significance, as determined by one‐way ANOVA, is P = 0.7412 (not significant). Quantification of proportion of macrophages that are tnfα‐positive inflammatory macrophages at the wound site at 8 DPI, measured from images represented in (F). N = 12 independent fish per timepoint per condition. Statistical significance, as determined by one‐way ANOVA, is P ≤ 0.0001. Quantification of total blood vessel length at 10 DPI, measured from images represented in (F) using Angioanalyser. N = 12 independent fish per timepoint per condition. Statistical significance, as determined by one‐way ANOVA, is P ≤ 0.0001. Gene expression for FAC‐sorted Tg(mpeg:mCherry); Tg(tnfα:GFP) zebrafish wound macrophages extracted at 7 DPI following needle‐stick injury. tnfα‐positive inflammatory macrophages express higher levels of vegfaa than tnfα‐negative non‐inflammatory macrophages. N = 100 independent fish per condition, 3 replicates. Statistical significance is indicated, as determined by two‐tailed t‐test. |
|
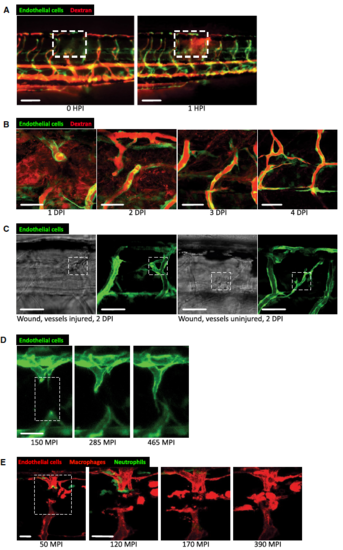
Dynamic vessel sprouting in response to tissue damage results in re-establishment of vessel patency within four days in zebrafish wounds. A Representative fluorescent stereomicroscope images taken from 30-gauge needle-stick wounded Tg(fli:GFP) zebrafish. High molecular weight dextran was loaded one hour prior to injury, with images taken immediately after and one hour after injury, showing leakage of dextran (red) into the wound site upon vessel damage. N = 12 independent fish. B Confocal projection images of a representative 30-gauge needle wounded Tg(fli:GFP) zebrafish, loaded with dextran one hour prior to imaging, showing the typical time course of blood vessel leakiness over 4 DPI following needle-stick injury as per (A). Vessels typically cease leaking dextran by 4 DPI. N = 12 independent fish per timepoint. C Confocal projection images of a representative fine tungsten needle wounded Tg(fli:GFP) zebrafish, with damage induced to an existing vessel or between existing vessels. DIC images are provided as a guide to injury positioning. N = 8 independent fish per condition. D Representative confocal projection images taken from timelapse movies of a laser injured Tg(fli:GFP); Tg(mpx:GFP) zebrafish, 4 DPF, imaged at 150–465 MPI, showing the dynamic nature of vessel tip extensions. N = 10 independent fish. E Representative confocal projection images taken from timelapse movies of a laser injured Tg(kdrl:mCherry-CAAX); Tg(mpx:GFP); Tg(mpeg:mCherry) transgenic zebrafish, 4 DPF, imaged at 30–420 MPI (Movie EV5), to complement our dynamic studies of neutrophils and macrophages observed in Movie EV2. N = 5 independent fish. |
|
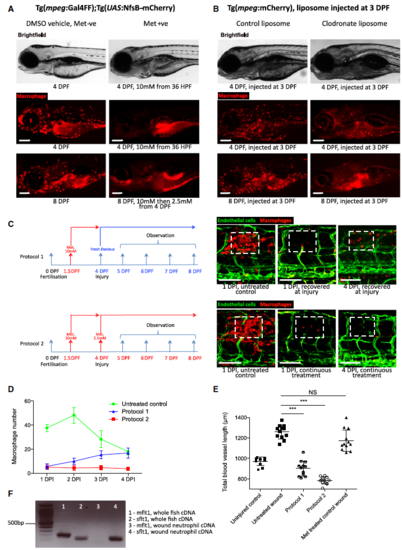
Extent of wound angiogenesis is proportional to number of pro-angiogenic macrophages versus anti-angiogenic neutrophils present at wound site. A Representative fluorescent stereomicroscope images taken from Tg(mpeg:KalTA4); Tg(UAS:nfsB-mCherry) transgenic zebrafish, either treated with control DMSO or metronidazole as indicated. Metronidazole treatment resulted in > 90% macrophage ablation using this genetic model. N = 10 independent fish per condition. B Representative fluorescent stereomicroscope images taken from Tg(mpeg:mCherry) transgenic zebrafish, injected with either control liposomes or clodronate liposomes, showing similar levels of macrophage ablation to metronidazole treatment. N = 10 independent fish per condition. C Two metronidazole treatment protocols were used to ablate macrophages prior to injury, or throughout injury. Wounded Tg(mpeg:KalTA4); Tg(UAS:nfsB-mCherry); Tg (fli:GFP) transgenic zebrafish larvae were treated with DMSO (control) or metronidazole as indicated. Boxed area denotes wound site. D Quantification of number of macrophages at the wound site of DMSO-treated control fish versus fish treated under the two ablation protocols, measured from images represented in (C) and Fig 2. Under protocol 1, fish recovered into fresh water post-injury saw a partial rescue of macrophage numbers at the wound site by 4 DPF. N = 12 independent fish per condition per timepoint. E Quantification of total blood vessel length, measured from images represented in (C), using Angioanalyser. Under protocol 1, fish with partially rescued macrophages also showed a trend towards increased wound angiogenesis compared to full macrophage ablation. Treatment of Tg(fli:GFP) transgenic zebrafish with metronidazole showed no difference in extent of wound angiogenesis compared to untreated injury. N = 12 independent fish per condition per timepoint. Statistical significance, as determined by one-way ANOVA, is P ≤ 0.0001. Subsequent Bonferonni multiple comparison test, determines level of significance, as indicated. Significance values: ***P ≤ 0.0001. F 1% agarose gel, showing that both mflt1 and sflt1 products are present in whole zebrafish cDNA, but only sflt1 cDNA is present in FAC-sorted Tg(mpx:GFP) neutrophils in early zebrafish wounds, as outlined in Fig 3F. |
|
Manipulation of normally dynamic macrophage phenotype towards a pro-inflammatory state alters the extent of wound angiogenesis and the patency of vessels. A Representative confocal projection images taken from either 1 DPI Tg(mpeg:mCherry); Tg(tnfa:GFP) transgenic zebrafish (top panel) or 4 DPI Tg(fli:GFP) transgenic fish (bottom panel), wild type or csf1ra/ mutant, treated as indicated with LPS or hydrocortisone. Boxed area denotes wound site. B Quantification of number of macrophages at the wound site from 1 to 4 DPI, measured from images represented in (A) and Fig 4. N = 14 independent fish per timepoint per condition. C Quantification of proportion of macrophages that are tnfa positive at the wound site from 1 to 4 DPI, measured from images represented in (A) and Fig 4. N = 14 independent fish per timepoint per condition. D Quantification of total blood vessel length at 4 DPI, measured using Angioanalyser from images represented in (A). N = 14 independent fish per timepoint per condition. Statistical significance, as determined by one-way ANOVA, is P ≤ 0.0001. Subsequent Bonferroni multiple comparison test, determines level of significance, as indicated. Significance values: *P ≤ 0.05, **P ≤ 0.001. E Representative confocal projection images of 30-gauge needle wounded Tg(fli:GFP) zebrafish larvae, treated with DMSO, LPS or Ifn-c from moment of injury, loaded with dextran (red) one hour prior to imaging, imaged at 4 DPI. Boxed area indicates wound site. F Representative confocal projection images taken from laser wounded Tg(kdrl:mCherry-CAAX); Tg(mpeg:mCherry); Tg(tnfa:GFP) transgenic zebrafish, imaged 30– 480 MPI. Similar early recruitment was observed with respect to tnfa-positive macrophages (arrowheads) versus tnfa-negative macrophages (asterisks), complementing our results from Movie EV8. As time progresses, some tnfa-positive macrophages diminish their GFP expression as they leave the site of injury (arrow). Boxed area denotes wound site. N = 5 independent fish. |
|
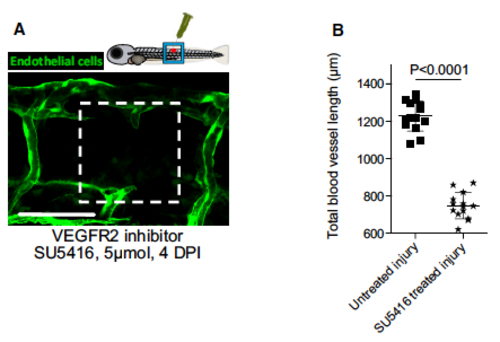
Suppression of vegf signalling causes failure of wound angiogenesis in zebrafish. A Representative confocal projection image taken from needle-stick wounded Tg(fli:GFP) transgenic zebrafish, imaged 4 DPI and treated with vegfr2 inhibitor SU5416 from the moment of injury. Boxed area denotes wound site. Scale bar, 100 lm. B Quantification of total blood vessel length, measured from images represented in (A) using Angioanalyser. N = 10 independent fish. Statistical significance is indicated, as determined by two-tailed t-test. Error bars indicate mean SD. |