- Title
-
Key Features of Structural and Functional Organization of Zebrafish Facial Motor Neurons Are Resilient to Disruption of Neuronal Migration
- Authors
- McArthur, K.L., Fetcho, J.R.
- Source
- Full text @ Curr. Biol.
|
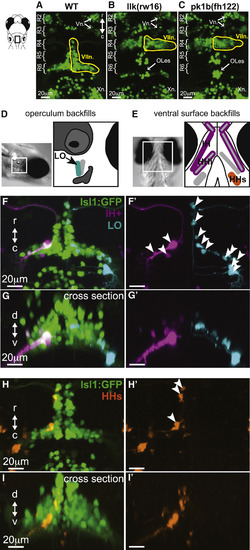
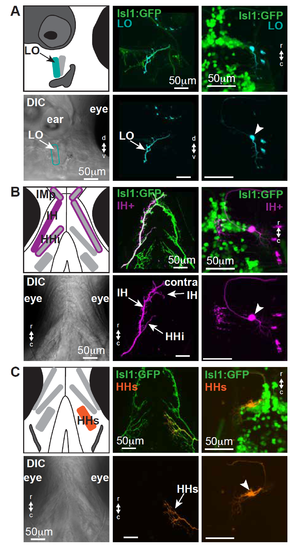
Facial Motor Neurons Migrate Caudally in Wild-Type, but Not Migration Mutant, Larvae, and Backfills from Cranial Muscles Reveal the Location of Facial Motor Pools Top panels show the hindbrain of Tg(Islet1:GFP) larvae at 5 dpf. (A) In wild-type larvae, facial motor neurons are born in rhombomere 4 (R4) and migrate caudally to settle in rhombomeres 5–7 by 48 hpf. (B and C) In both llk(rw16) mutant larvae (B) and pk1b(fh122) mutant larvae (C), facial motor neurons fail to migrate caudally and remain in R4. Octavolateralis efferents (OLes) born in R4 and R6 also fail to migrate caudally. (D and E) Schematic representation of the operculum (D) and ventral cranial muscles (E) targeted for backfills of the facial motor nerve. Filled muscles indicate the electroporation target, and outlined muscles correspond to other muscles labeled with dye after target electroporation. (F and G) Electroporation of fluorescent dyes into the IH (magenta) and LO (cyan) muscles on opposite sides of a single zebrafish larva (4 dpf) backfills a subset of each muscle's facial motor pool, imaged at 5 dpf, and shown here from dorsal (F and F') and cross-sectional (G and G') views. (H and I) Targeting the electroporation to the HHs (orange) backfills a subset of that muscle's motor pool, shown here from dorsal (H and H') and cross-sectional (I and I') views. Arrowheads indicate backfilled cell bodies. HHs, hyohyoideus superior; IH+, interhyoideus + hyohyoideus inferior; LO, levator operculi; Vn., trigeminal motor nuclei; VIIn., facial motor nucleus; Xn., vagal motor nucleus. See also Figure S1. |
|
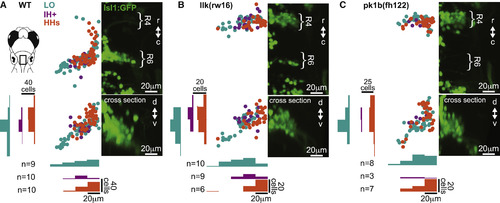
Facial Motor Pools Exhibit a Loose Spatial Topography that Is Maintained in Caudal Migration Mutants (A) Across wild-type larvae, the LO motor pool (teal) extends throughout the entire facial motor nucleus, whereas the IH+ (purple) and HHs (orange) motor pools are found in more restricted but still overlapping regions—in the middle and dorsomedial portions of the nucleus, respectively. (B and C) In llk(rw16) mutant larvae (B) and pk1b(fh122) mutant larvae (C), the cross-sectional topography of the motor pools is maintained ( Table S1). All fish were backfilled at 4 dpf and imaged at 5 dpf. n, number of fish used in each condition. Histograms illustrate the distribution of cells along the mediolateral and dorsoventral axes, binned at 20-μm intervals. See also Figure S1. |
|
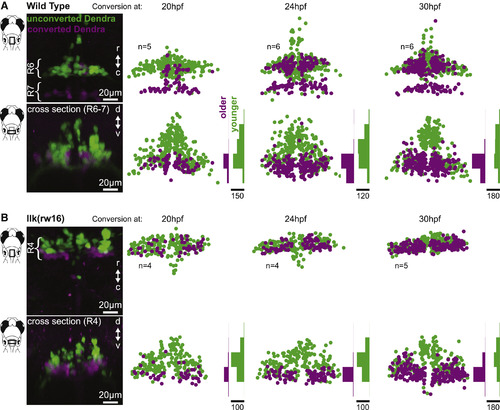
Facial Motor Neurons Are Topographically Organized According to Age in Both Wild-Type and Caudal Migration Mutants Zebrafish embryos expressed the photo-convertible protein Dendra2 in cranial motor neurons and were exposed to UV light at one of three time points during facial motor neuron differentiation (20, 24, or 30 hpf) and then imaged at 3 dpf. Neurons older than the conversion time point contain converted (magenta/purple) Dendra, whereas younger neurons contain only unconverted (green) Dendra. In both wild-type (A) and llk(rw16) mutant (B) larvae, older facial motor neurons are located in the ventral-most part of the facial motor nucleus. Examples in left panels of (A) and (B) have been color adjusted to increase visibility of the converted (magenta) Dendra signal. Histograms illustrate the distribution of cells along the dorsoventral axis binned at 20-μm intervals, where the scale bars refer to the number of cells. See Figure S2 for data from pk1b(fh122) mutants. |
|
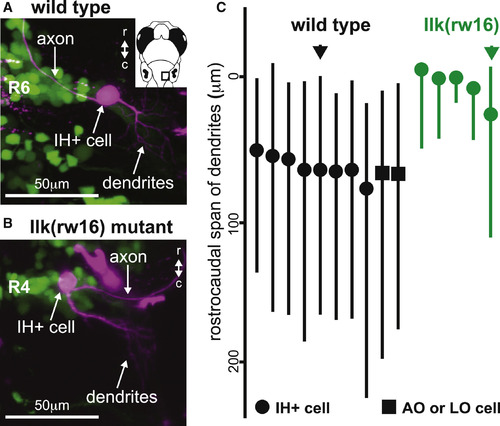
The Location of Dendrites from Facial Motor Neurons in Wild-Type and Migration Mutants (A and B) In both wild-type (A) and llk(rw16) mutant (B) larvae, single-cell dye fills show that FBMNs extend their dendrites primarily into the neuropil caudal and lateral to the cell body. (C) However, a comparison of the rostrocaudal span of FBMN dendrites reveals that wild-type FBMNs innervate more caudal regions of the neuropil than migration mutant FBMNs. Circles, IH+-projecting FBMNs; squares, operculum-projecting FBMNs. Black and green arrows correspond to the neurons used in (A) and (B), respectively. All cells were filled at 4 dpf and imaged at 5 dpf. |
|
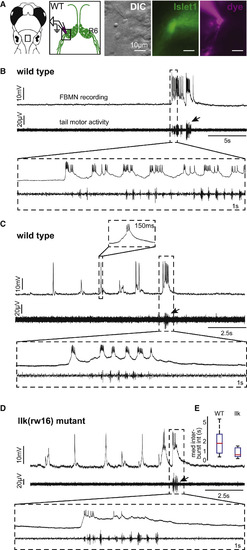
Whole-Cell Recordings Show that Rhythmic and Swim-Related Activity Exhibited by Wild-Type FBMNs Is Also Present in Migration Mutant FBMNs (A) Whole-cell patch-clamp recordings were made from the ventrolateral arm of the facial motor nucleus. (B) These recordings reveal a subset of wild-type FBMNs (n = 8/23 cells) that burst infrequently, correlated with tail motor activity. (C) Other wild-type FBMNs also exhibit rhythmic bursting, typically in the absence of tail motor activity (n = 15/23 cells). (D) Migration mutant llk(rw16) FBMNs can also respond during attempted tail movements (infrequent bursts only: n = 2/8 cells) and in rhythmic bursts without large tail motor activity (n = 6/8 cells). All recordings were done at 5 dpf. Arrows indicate bursts of attempted tail movement activity. (E) Box-whisker plot showing the distribution of median rhythmic inter-burst intervals in wild-type (n = 15 cells) and llk(w16) mutant (n = 6 cells) larvae. |
|
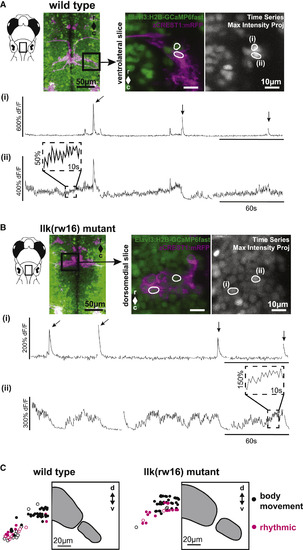
Calcium Imaging in Intact Larvae Confirms Patterns of FBMN Activity and Reveals a Robust Functional Spatial Topography (A) In wild-type larvae, some FBMNs exhibit only large, infrequent calcium transients during attempted body movements, whereas other FBMNs exhibit additional rhythmic activity. These two response types could be observed in neighboring FBMNs, as shown here. Outlined regions of interest (i and ii) correspond to the calcium traces shown in (i) and (ii). Sampling interval = 247 ms. (B) FBMNs in llk(rw16) migration mutants also exhibit these two activity patterns. Sampling interval = 493 ms. Arrows in (A) and (B) indicate large body movements. (C) In both wild-type (left) and llk(rw16) mutant (right) larvae, there was significant overlap of neurons exhibiting infrequent only (black symbols) and rhythmic (magenta symbols) activity, though rhythmic neurons were concentrated ventrolaterally (Table S2). Open symbols, neurons backfilled from the LO (operculum). Grey panels show the cross-sectional outline of the facial motor nucleus for each phenotype, based on backfill data shown in Figure 2. Number of fish used to assemble summary data: n = 7 wild-type (64 classified neurons in total); n = 6 llk(rw16) mutant (60 classified neurons in total). All data shown here were obtained from unparalyzed fish at 5 dpf. |
|
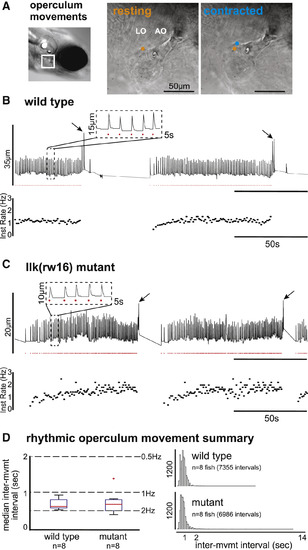
Wild-Type and Migration Mutant Larvae Exhibit Rhythmic Opercular Movements with Similar Temporal Properties (A) Operculum rotations were manually recorded from restrained larvae by tracking the position of landmarks over time across resting (orange) and contracted (blue) states (sampling frequency = 20 Hz). (B and C) For each example (B and C), vertical landmark position is plotted over time (top trace), with rhythmic operculum movement times indicated by red dots and instantaneous movement frequency plotted below. Both wild-type (B) and llk(rw16) mutant (C) larvae exhibit bouts of rhythmic operculum rotation. (D) The box-whisker plot (left) shows the distribution of per-fish median inter-movement intervals in wild-type (n = 8) and mutant (n = 8) larvae. The histograms (right) show the complete distribution of inter-movement intervals in wild-type (top; n = 7,355 intervals) and mutant (bottom; n = 6,986 intervals) larvae. Migration phenotype was not a significant predictor of inter-movement interval (p = 0.613; see Table S3). |
|
In zebrafish larvae, some facial motor neurons innervate a single muscle, while others innervate multiple muscles; see also Figures 1 and 2. In each section (A-C), a schematic of the cranial musculature is shown in the upper left, with a corresponding DIC view in the lower left. The axon terminal from a single-cell is shown in the middle (upper and lower) panels, with the corresponding filled cell body shown in the right (upper and lower) panels. (A) Both backfills and single-cell fills are consistent with the existence of an LO-only facial motor pool. Electroporation targeting the LO facial nerve branch did not fill other nerve branches, and the axons of filled neurons projecting to the LO do not also terminate on other muscles. (B) Both backfills and single-cell fills are consistent with the existence of a multi-muscle motor pool innervating both ipsilateral and contralateral IH plus the ipsilateral HHi and the bilateral IMp (a muscle typically associated with trigeminal innervation). As shown here, a single facial motor neuron axon can terminate on all of these muscles. Some of these muscles will fuse later in development [S1]. (C) Both backfills and single-cell fills are consistent with the existence of an HHs-only facial motor pool, though this muscle will separate into two separate muscles later in development [S1]. Electroporation targeting the HHs facial nerve did not fill other nerve branches, and the axons of filled neurons projecting to the HHs do not typically terminate on other muscles. Rarely, a small branch might be seen to extend onto the IH, but these small branches were not elaborated and might not be functional. LO = levator operculi, IH = interhyoideus, HHi = hyohyoideus inferior, HHs = hyohyoideus superior, IMp = intermandibularis posterior. |
|
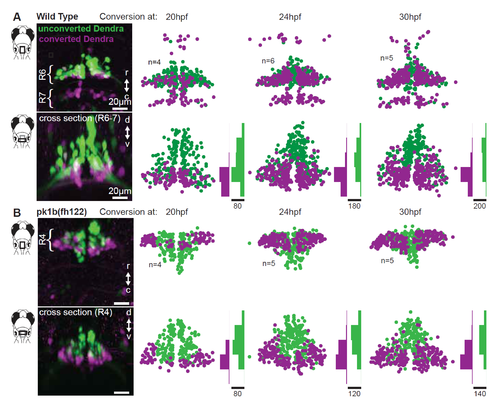
Facial motor neuron age topography in wild type and pk1b(fh122) mutants. Related to Figure 3. Zebrafish embryos expressed the photo convertible protein Dendra in cranial motor neurons and were exposed to ultraviolet light at three time points during facial motor neurons differentiation (20, 24 and 30 hpf), then imaged at 3 dpf. For each conversion time, neurons older than that time point contain converted (magenta/purple) Dendra, while younger neurons contain only unconverted (green) Dendra. (A) In wild type larvae, older facial motor neurons are located in the ventral-most part of the facial motor nucleus. (B) In pk1b(fh122) mutant larvae, facial motor neurons adopt the same dorsoventral age topography observed in wild type larvae. Note that there is a modest shift in the earliest conversion time point, such that the oldest mutant neurons are found laterally as well as ventrally. |