- Title
-
Impaired embryonic motility in dusp27 mutants reveals a developmental defect in myofibril structure
- Authors
- Fero, K., Bergeron, S.A., Horstick, E.J., Codore, H., Li, G.H., Ono, F., Dowling, J.J., and Burgess, H.A.
- Source
- Full text @ Dis. Model. Mech.
|
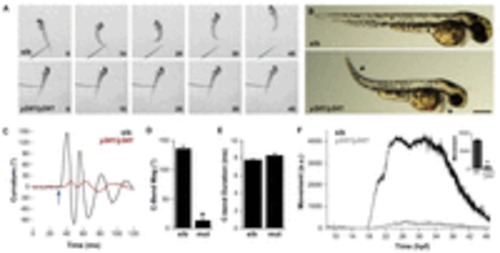
Embryonic motility is impaired in y241 mutant embryos. (A) Swimming response to a tactile stimulus is severely impaired in 2 dpf y241 mutants. Image frames are at 10 ms intervals from a touch stimulus delivered with a fine needle. Siblings (top panels) swim rapidly forward, whereas mutants (lower panels) respond with a barely perceptible shiver. (B) y241 mutants (bottom panel) show edema adjacent to the heart (asterisk), tail curvature (arrowhead) and smaller eyes. Scale bar: 300 μm. (C) Curvature trace for 120 ms during an acoustic startle response in a sibling (black) and y241 mutant (red) embryo. The stimulus was delivered at 30 ms (arrow). (D) The magnitude of the initial C-bend in y241 mutant acoustic startle responses is reduced compared with that of wild-type embryos (n=6 larvae each), whereas (E) the duration of the initial C-bend is not significantly different. *P<0.001. (F) Spontaneous coiling is strongly reduced in y241 mutants (gray, n=7) compared with wild-type siblings (black, n=37) during the entire period of early locomotor development. y-axis is arbitrary units. Inset, average coiling during 24–28 hpf for the same larvae. *P<0.001. Graphs show mean ± s.e.m. PHENOTYPE:
|
|
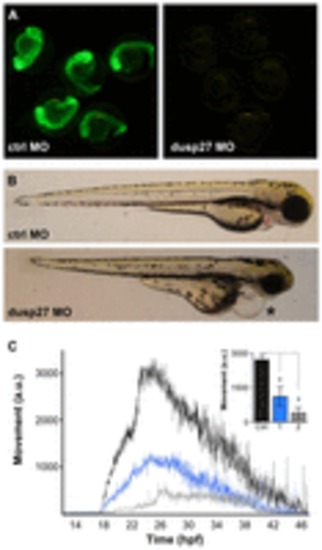
Morpholino knockdown of dusp27 phenocopies y241 mutants. (A) Validation of morpholino efficacy for dusp27 knockdown using a dusp27 N-terminal sequence fused to GFP. (B) Morphology of embryos injected with control morpholino (top panel) or 1 ng of morpholino against dusp27 (bottom panel). Embryos show pronounced edema (asterisk). (C) Spontaneous coiling in embryos injected with control morpholino (black, n=11), 1 ng dusp27 morpholino (blue, n=9) or 2 ng dusp27 morpholino (gray, n=9). Inset, average coiling during 24–28 hpf for the same larvae. *P<0.01. Graphs show mean ± s.e.m. PHENOTYPE:
|
|
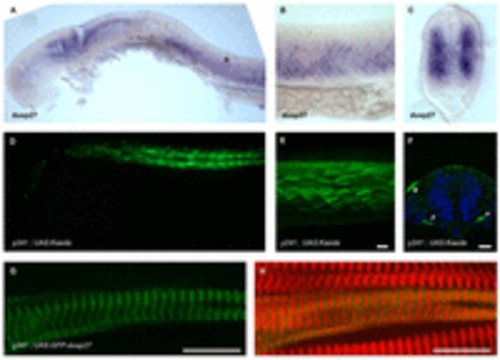
dusp27 is expressed during embryonic muscle development. (A–C) In situ hybridization for dusp27 expression at 24 hpf. Prominent expression is seen in the somites (asterisk). (B) Lateral view above the yolk extension. (C) Cross-section through the trunk caudal to the yolk extension. (D–F) Kaede reporter expression in y241; UAS:kaede embryos. (D) Kaede expression is strongly expressed in trunk muscle at 24 hpf and (E) continues to label myofibers in 6 dpf larvae. Scale bar: 50 μm. (F) Early expression in somites is observed at 15 somites in a small number of cells adjacent to the notochord (arrowheads), as well as at the lateral edge of the somite (asterisk). Scale bar: 50 μm. (G,H) Expression of GFP-Dusp27 in muscle cells shows a striated pattern (green, G) that alternates with α-actinin (red in merge, H). Scale bars: 10 μm. EXPRESSION / LABELING:
|
|
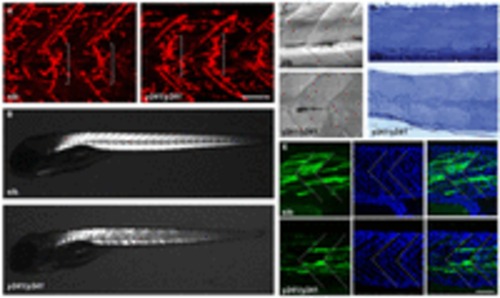
Muscle integrity is impaired in y241 mutants. (A) α-bungarotoxin-conjugated Alexa-Fluor-555 staining of 2 dpf wild-type sibling (left) and y241 mutant (right) embryos, showing equatorially located neuromuscular junctions (brackets). Scale bar: 50 μm. (B) Birefringence in live 5 dpf wild-type sibling (top) and y241 mutant (bottom) larvae detected using polarized light. Mutants show reduced and patchy birefringence. (C) Dodt-gradient-contrast illumination of muscle in 3 dpf larvae after removal of skin. Internal muscle striations are visible in myofibers stretching between somite boundaries in siblings (top) but not in y241 mutants (bottom). (D) 10 μm toluidine-blue-stained sections through the tail of 3 dpf wild-type siblings (top) and y241 mutants (bottom). (E) Kaede expression in highly variegated 24 hpf y241; UAS:kaede sibling embryos (top panels) and mutants (bottom panels) with DAPI staining to reveal nuclear positions. Somite boundaries observed in bright-field images are indicated by dashed lines. Scale bar: 30 μm. |
|
Slow muscle myofibrils are partially disrupted in y241 mutants. (A) F59 staining against slow-twitch muscle myosin heavy chain in 48 hpf embryos shows the presence of slow muscle fibers at the lateral edge of the somite in both mutants and siblings but disorganized thick filaments in y241 mutants. Scale bar: 20 μm. Insets show rotated transverse view through the somite. (B) α-actinin staining in slow-twitch fibers shows preserved Z-lines (arrowheads) in y241 mutants. Scale bar: 25 μm. |
|
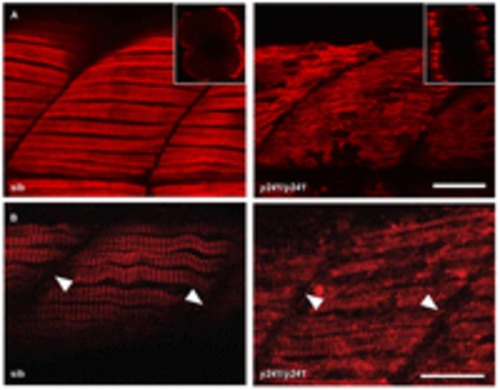
Fast muscle myofibrils are disrupted in y241 mutants. (A–E) Immunofluorescence in fast-twitch fibers in 48 hpf sibling and y241 mutant embryos. (A) F310 labeling of fast-twitch-fiber myosin shows disruption of thick filaments. Scale bar: 25 μm. Insets show rotated transverse view through the somite (merged view of DAPI in blue and F310 in red). (B) CH1 labeling of tropomyosin shows disruption of thin filaments. Scale bar: 20 μm. (C) α-actinin staining shows loss of Z-lines in fast-twitch fibers. Scale bar: 25 μm. (D) Anti-titin staining. Scale bar: 30 μm. (E) DHPR in fast-twitch fibers. Scale bar: 25 μm. (F) Transmission electron microscopy in myofibers of 3 dpf y241 siblings and mutants, showing Z-lines (arrows) and triads (arrowheads). Scale bars: 500 nm. EXPRESSION / LABELING:
PHENOTYPE:
|

Measurement of embryonic motor activity. (A) Schematic of the apparatus used to record motor activity in embryos. Embryos are arrayed in a 49 well grid in a Petri dish with temperature maintained at 28°C using a thermistor and heating element. A camera mounted above records images (B) at a 1 Hz framerate. (C) Motor activity is measured as the sum of the number of pixels which change in intensity greater or less than a predetermined threshold between frames. Top row shows a sequence of frames for the boxed well in (B), while the bottom row shows the same frames, with changed pixels highlighted and the total number of changed pixels indicated. To allow real-time processing, only every second pixel in the image is checked. (D) For validation, the coiling frequency was manually observed for 20 embryos (number of coiling events in a one minute interval) and the total number of pixel change events automatically measured for the same video sequence. Manual and automatically scored values are highly correlated Pearson r=0.86, P<0.001 confirming that the algorithm captures differences in motor activity. (E) For additional validation, embryos were exposed to varying concentrations of tricaine for 20 minutes (N=14 each condition) before movement was measured over a 10 minute period. The y-axis shows the mean number of pixel changes per minute averaged over the recording period. 0.002% tricaine significantly reduced but did not eliminate movement while 0.02% tricaine completely paralyzed embryos such that the number of pixel changes was not significantly (n/s) different from baseline as measured in wells with no embryos. * P<0.001. |
|
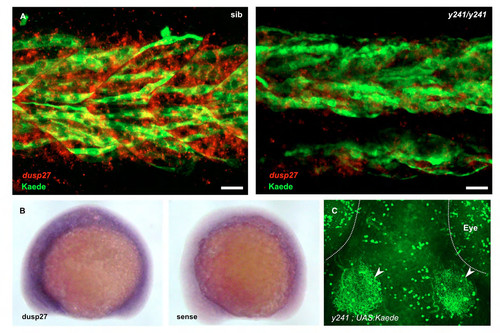
Additional analysis of dusp27 expression. (A) Muscle fibers labeled by anti-kaede staining (green) in y241 ; UAS:Kaede heterozygotes (left) at 30 hpf co-express dusp27 (red, in situ hybridization), while dusp27 expression in mutants is strongly diminished. Note that the expression of dusp27 in fibers not labeled by kaede is likely due to variegated expression of the UAS:Kaede transgene, a commonly observed phenomenon in zebrafish UAS lines (Goll et al., 2009). Scale bar 20 μm. (B) in situ hybridization for dusp27 at 15 somites (right panel shows sense control) (C) In 6 dpf brain, Kaede prominently labels deep tectal neurons that extend neurites into the tectal neuropil (arrowheads) as well as neuronal groups in the retina, cerebellum and hypothalamus (not shown). Scale bar 50 μm. |
|
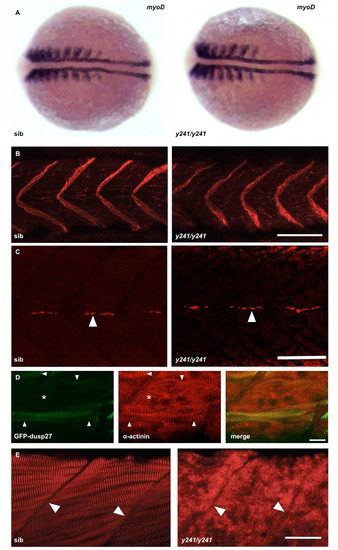
Additional analysis of muscle markers in y241 mutants. (A) In situ hybridization for myoD expression in 10 somite sibling (left) and y241 mutant (right) embryos. After photographing, embryos were genotyped by PCR. (B) Immunofluorescence staining for dystrophin (merged z-stack through muscle) shows similar localization at somite boundaries in sibling and y241 mutants in 48 hpf embryos. Scale bar 50 μm. (C) 4D9 staining of engrailed expression in muscle pioneer cells indicates the presence of grossly normal densities of pioneer cells (arrows). Scale bar: 50 μm. (D) In y241 mutants injected with the GFP-dusp27 plasmid, muscle fibers expressing GFP (left panel, arrowheads) recover a normal striated pattern of α-actinin expression (middle panel) while regions of the somite without GFP expression do not show patterned α-actinin expression (asterisk). Merged view on right. Scale bar 10 μm. (E) The normal striated pattern of ryanodine receptor staining is disrupted in 48 hpf y241 mutant embryos. Arrowheads point to somite boundaries. Scale bar 25 μm. |
|
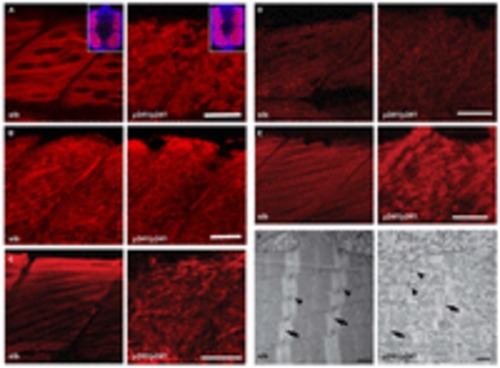
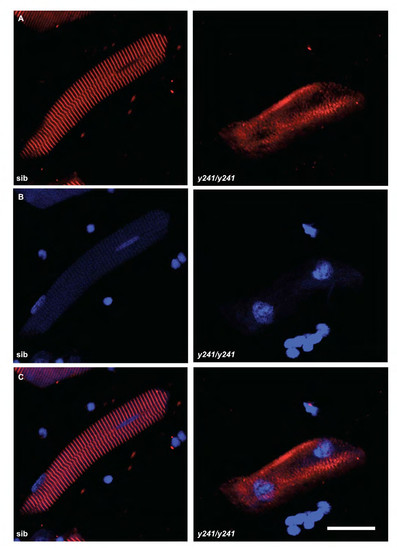
Immunofluorescence in isolated fast muscle fibers in y241 mutants. (A) α-actinin stain and (B) DAPI stain in isolated myofibers from 4 dpf sibling and y241 mutants. Merged image in (C). Scale bar 20 μm. |