- Title
-
Disruption of the folate pathway in zebrafish causes developmental defects
- Authors
- Lee, M.S., Bonner, J.R., Bernard, D.J., Sanchez, E.L., Sause, E.T., Prentice, R.R., Burgess, S.M., and Brody, L.C.
- Source
- Full text @ BMC Dev. Biol.
|
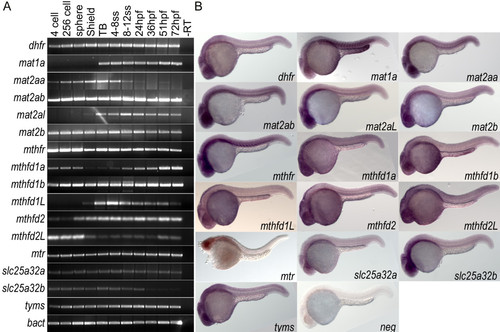
Wild type expression data for folate pathway transcripts. A) RT-PCR analysis of 11 embryonic stages within the first 72 hours of development indicates that genes involved in the folate pathway are generally maternally loaded transcripts that are expressed throughout early development. B) in situ hybridization for these 16 genes at 24 hpf shows that many are globally expressed with enrichment in anterior neural tissues including tectonic and retinal staining. Specific somatic staining is seen in mat1a, mat2aa, mat2ab, and mthfr. YSL staining is also observed in mat1a and mthfd1b. Embryos in lateral view. EXPRESSION / LABELING:
|
|
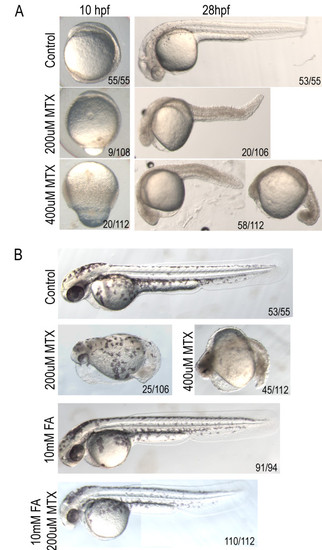
Methotrexate treatment confers dose-dependent developmental delay. A) Treatment with lower concentrations of MTX causes shortening of the anterior-posterior axis and heart defects, while higher doses result in embryonic lethality prior to somitogenesis. B) Defects evident at 2 dpf in embryos treated with 200 μM methotrexate are alleviated by treatment with 10 mM Folinic Acid (FA). Embryos in lateral view. |
|
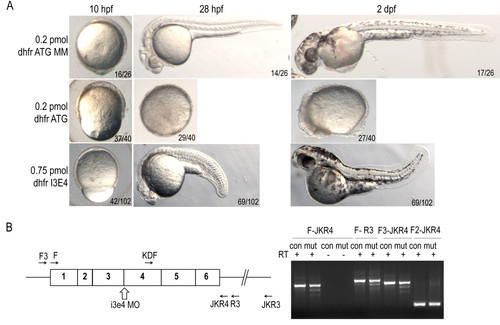
dhfrmorpholino injection phenocopies methotrexate treatment defects. A) Epiboly in dhfr ATG morphants is significantly delayed at 10 hpf as compared to controls. By 28 hpf severe dhfr ATG morphants are developmentally arrested with a failure to complete epiboly. Intron-exon splice morphants complete epiboly but exhibit shortened anterior-posterior axis and heart defects. Embryos in lateral view. B) Diagram of dhfr mRNA, i3e4 splice blocking morpholino, and primers used for RT-PCR (sequences supplied in Additional file 2: Table S2). cDNA generated from embryos at 6 hpf demonstrate splicing defects in dhfr transcripts in embryos treated with morpholino (mut). Control injected embryos (con) have only expected band sizes. |
|
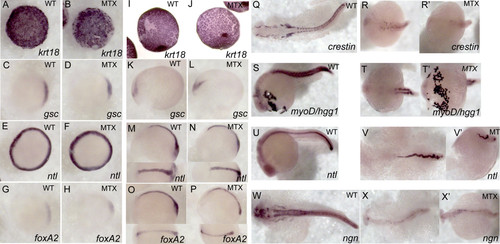
Methotrexate–treated embryos maintain expression of appropriate developmental markers. Expression patterns were visualized via whole-mount in situ hybridization in embryos treated with 400 μM MTX and fixed at shield stage (A-H), tailbud (I-P), and 24 hpf (Q-W′). While MTX-treated embryos at 24hpf are clearly delayed with respect to normal development, they do express appropriate developmental markers: krt18 –enveloping layer, gsc – involuting cells and neural plate, ntl – blastomere margin, notochord and dorsal organizer, foxA2 – involuting cells and neural plate, crestin – neural crest, myoD – paraxial mesoderm, hgg- hatching gland (anterior most structure), ngn – CNS. (A-J) Animal view, dorsal to the right. (K-P) Lateral view with dorsal view inset. (Q, R, R′, T, V, W, X, X′) Dorsal view. (S, U, V′) Lateral view. (T′) ventral view. |
|
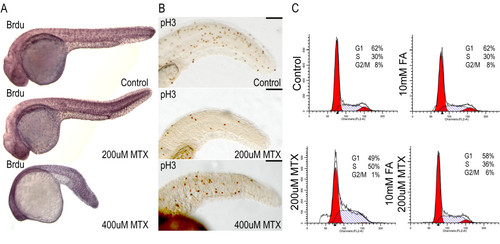
Abnormal cell cycle properties of methotrexate treated embryos A) After 24 hours of 200 μM or 400 μM methotrexate exposure, embryos are capable of incorporating BrdU via DNA synthesis. B) Immunohistochemistry for the mitotic marker phospho-histone H3 shows a decreased number of mitotic cells in methotrexate treated embryos. C) However, FACS analysis clearly indicates methotrexate treated embryos do not display WT cell cycling profiles, with a probable S-phase accumulation. Co-treatment with folinic acid and methotrexate restores cell cycle profiles. Lateral views of 24 hpf embryos. Scale bar: 100 μm. |