- Title
-
In vivo evidence for transdifferentiation of peripheral neurons
- Authors
- Wright, M.A., Mo, W., Nicolson, T., and Ribera, A.B.
- Source
- Full text @ Development
|
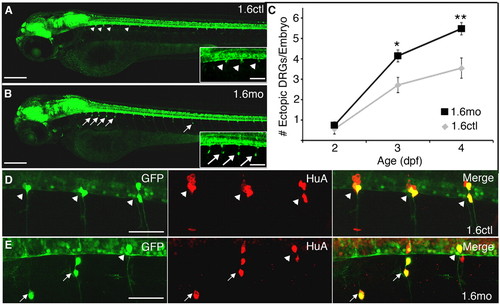
DRG neurons reside in abnormal positions in nav1.6 morphants. (A) GFP+ DRG neurons show a stereotypic pattern and localize to the ventral boundary of the spinal cord (arrowheads) in each trunk hemisegment of control 3 dpf Tg(ngn:GFP) embryos. (B) By contrast, many GFP+ DRG cells reside in more ventral locations (arrows) in 1.6 morphants. Insets in A and B show segments 2-5 at higher magnification. (C) In both controls (gray diamonds) and morphants (black squares) the average number of ectopic DRG neurons per embryo increases between 2 and 4 dpf. Additionally, a larger number of ectopic DRG neurons are found in morphants than controls at 3 and 4 dpf (*P<0.01 versus 1.6mo; **P<0.001 versus 1.6mo; Kruskal-Wallis nonparametric ANOVA). (D) In 4 dpf control embryos, DRG neurons (arrowheads) express both GFP (green) and HuA (red), a marker of neuronal differentiation. (E) Ectopic DRG neurons (arrows) are also positive for both GFP and the neural differentiation marker, HuA. Embryos are mounted laterally, with anterior towards the left and dorsal at the top for all figures. Scale bars: 200 μm in A,B (70 μm in insets); 50 μm in D,E. EXPRESSION / LABELING:
PHENOTYPE:
|
|
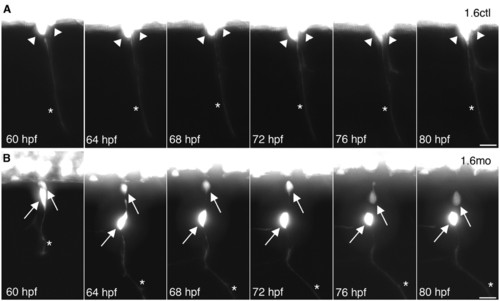
DRG neurons migrate to abnormal ventral positions after they have extended axons. (A) Two adjacent DRG neurons (arrowheads) in a Tg(–3.4neurog1:GFP) control embryo extended axons (asterisk) by 60 hpf. Between 60 and 80 hpf, control DRG neurons maintain their position and remain lateral to the ventral spinal cord. Data are representative of 16 control DRG that were followed by time-lapse imaging. (B) In a 60 hpf Tg(–3.4neurog1:GFP) morphant embryo, DRG neurons (arrows) reside lateral to the ventral spinal cord and have extended axons (asterisks), similar to the control shown in A. However, at 64 hpf, one DRG neuron has migrated ventrally. By 76 hpf, the second DRG neuron has also migrated ventrally. Data are representative of 18 ventrally migrating morphant DRG neurons. Scale bars: 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
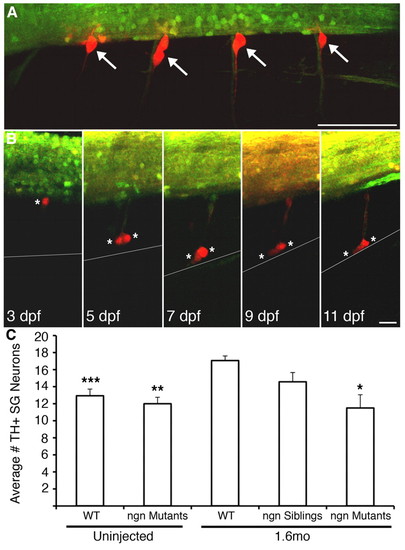
Migratory DRG migrate as far ventrally as the location of the sympathetic ganglia. (A) Transgenic expression of photoconvertible Kaede in DRG allows identification of progeny and migratory paths. At 60 hpf, DRG (white arrows) in four hemisegments of a nav1.6 morphant Tg(elavl3:Kaede) embryo were selectively illuminated with UV light, as evidenced by photoconversion of Kaede from a green fluorescent molecule to red in DRG, but not nearby spinal cord, neurons. (B) At 3 dpf, a single DRG neuron (white asterisk) shows a stereotypic location at the ventral boundary of the spinal cord. At 5 dpf, the cell divides into two cells (white asterisks), but retains its axon, then migrates ventrally. At 7 dpf, both cells approach the ventral boundary of the notochord (grey line), the location of the sympathetic ganglia. The two migratory DRG cells remain in the region of the sympathetic ganglia at subsequent times (e.g. 11 dpf). (C) Consistent with migration of DRG neurons to the location of the sympathetic ganglia, the number of TH+ neurons in the sympathetic ganglia at 11 dpf is significantly greater in 1.6 morphant embryos compared with wild type (***P<0.001 versus 1.6mo wild type, nonparametric ANOVA). However, injection of 1.6mo does not produce an increase in TH+ neurons in the sympathetic ganglia in neurogenin mutants, which lack DRG neurons (**P<0.01 versus 1.6mo wild type; *P<0.05 versus 1.6mo wild type; Kruskal-Wallis nonparametric ANOVA. Scale bars: 30 μm in A; 15 μm in B. EXPRESSION / LABELING:
PHENOTYPE:
|
|
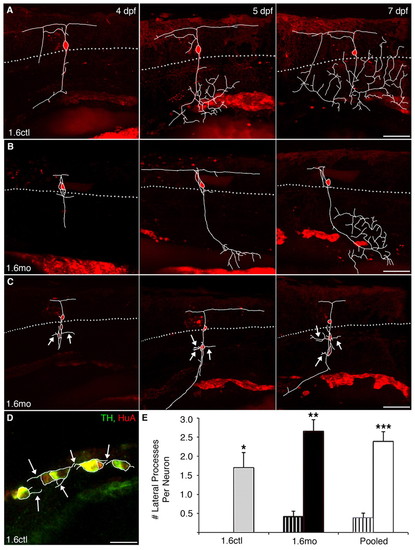
Migratory DRG neurons sprout novel lateral processes. DRG neurons were electroporated with Alexa568 at 4 dpf and followed to 7 dpf. In panels A-C, dye-labeled DRG processes were traced in white from individual slices and overlaid onto the projected images. The dotted gray line represents the ventral boundary of the spinal cord, where DRG are typically found. (A) In a control embryo, a DRG neuron extends a single process ventrally at 4 dpf. At subsequent times, the process begins to branch once it reaches more ventral positions. (B) In a nav1.6 morphant embryo, a correctly positioned DRG neuron also extends a ventral process that branches. (C) By contrast, ectopic DRG neurons of morphant embryos sprout processes (white arrows) that emanate from the soma and extend laterally. (D) In an 11 dpf control embryo, laterally projecting processes emerge from the soma of TH+/HuA+ sympathetic neurons (white arrows). (E) Normally positioned DRG neurons (striped bars), in either control (grey) or morphant (black) embryos, sprout few or no laterally projecting processes from their soma. By contrast, migratory DRG neurons (solid bars) display many more laterally projecting processes (*P<0.05 versus normally-positioned 1.6ctl; **P<0.001 versus normally-positioned 1.6mo; ***P<0.001 versus normally positioned pooled cells; Kruskal-Wallis nonparametric ANOVA). White bars represent pooled morphant and control cells. Scale bars: 60 μm in A-C; 20 μm in D. |
|
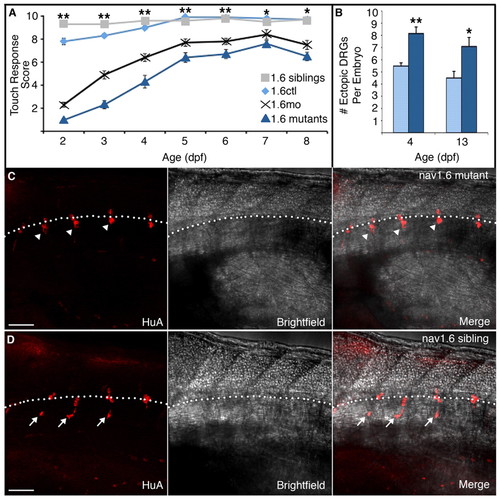
nav1.6 mutants recapitulate the morphant phenotype. (A) nav1.6 morphants (black crosses) and mutants (dark-blue triangles) show a severe reduction in touch sensitivity at 2 dpf compared with control (gray squares) and sibling (light blue diamonds) embryos. Although nav1.6 morphants and mutants partially recover by 5 dpf, they show similar deficits in touch sensitivity that persist as late as 8 dpf (**P<0.001 for nav1.6 siblings and controls versus nav1.6 mutants and morphants; **P<0.01 for nav1.6 siblings and controls versus nav1.6 mutants and morphants; Kruskal-Wallis nonparametric ANOVA). (B) The increase in the number of ectopic DRG neurons in nav1.6 mutant versus control larvae persists to 13 dpf (**P<0.0001, *P=0.0105; Mann-Whitney test). Scale bars: 50 µm. (C) Additionally, whereas HuA-positive DRG neurons cluster lateral to the ventral spinal cord in nav1.6 siblings (arrowheads), (D) many individual DRG neurons in nav1.6 homozygous mutants are located ectopically, in more ventral positions (arrows) reminiscent of DRG position in nav1.6 morphants. The broken white line indicates the ventral boundary of the spinal cord. |
|
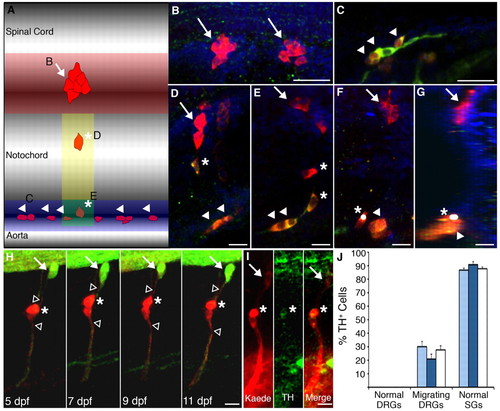
Migrating DRG neurons express TH, a marker of the sympathetic ganglia. (A-G) HuA+ neurons were classified as normally positioned DRG neurons (red shaded region; B, arrows), SG neurons (blue shaded region; C, arrowheads), or migratory DRG (yellow and green shaded regions; D-G, asterisks indicate migratory cells and arrowheads SG neurons). The yellow and green regions are both directly ventral to the normal location of the DRG; cells in the green region were classified as migratory DRG and not SG if they were positioned dorsal to nearby SG neurons and/or were elongated along the dorsoventral axis. (F) A projection of a confocal z-stack image shows a TH+ neuron identified as a migratory DRG neuron (asterisk, white dot), based upon its vertical orientation, adjacent to a sympathetic neuron (arrowhead). The projection consisted of 20 confocal slices obtained in increments of 3 μm. (G) Rotation of the projected image shows that the migratory DRG neuron (asterisk) resides within the sympathetic ganglia. (H) In Tg(elavl3:Kaede) larvae, we photoconverted two migratory DRG neurons (red, asterisk), identified by Kaede expression and the presence of dorsal and ventral axons (unfilled arrowheads) at 5 dpf, and followed them to 11 dpf. The white arrow indicates a normally positioned DRG that was not photoconverted and remains green fluorescent. (I) At 11 dpf, one of the cells was positive for both Kaede (red) and TH (green) immunoreactivities (asterisk). Overall, 25% of photoconverted DRG neurons (6/24; 15 embryos) showed TH immunoreactivity. (J) In 13-15 dpf larvae, normally positioned DRG neurons (B) rarely express TH. By contrast, the majority of SG neurons (C) do express TH. A significant number of migratory DRG neurons (D,E) express TH. However, the genotype of the embryo did not affect the percent of TH+ normally positioned DRG, SG or migratory DRG (light-blue bars, nav1.6 siblings; dark-blue bars, nav1.6 mutants; white bars, all embryos pooled regardless of genotype). (B-G) Embryos were immunostained for HuA (red) and TH (green); Hoescht (blue)-labeled nuclei. Scale bars: 20 μm in B,C; 10 μm in D-G; 12.5 μm in H,I. EXPRESSION / LABELING:
|
|
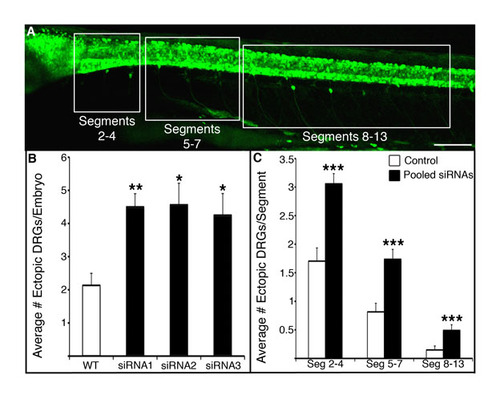
Knock-down of nav1.6a leads to migration of DRGs in the anterior and posterior trunk. (A,B) Three separate siRNAs targeting scn8aa increase the number of ectopic DRG neurons in 4 dpf Tg(ngn1:GFP) compared with uninjected embryos (*P<0.05 versus uninjected wild type; **P<0.01 versus uninjected wild type; Kruskal-Wallis nonparametric ANOVA). siRNAs were synthesized by Dharmacon (Lafayette, CO, USA) and had the following sequences: siRNA1, GGAGAATGGTGGTACTAAT; siRNA2, GGACCTGGCTATCACCATT; siRNA3, GGCCATGTGTCTCATCGTA. All three siRNAs reduce the touch response, similar to the 1.6 morpholino (not shown). (C) Although the majority of ectopic DRG neurons are found in segments 2-4 in wild type and siRNA-injected embryos, nav1.6a knock-down increases the number of ectopic DRG neurons in segments 2-4, 5-7 and 8-13 (***P<0.01 versus wild-type neurons in the same segments; two-tailed t-test). |