- Title
-
Phosphatase-Dependent and -Independent Functions of Shp2 in Neural Crest Cells Underlie LEOPARD Syndrome Pathogenesis
- Authors
- Stewart, R.A., Sanda, T., Widlund, H.R., Zhu, S., Swanson, K.D., Hurley, A.D., Bentires-Alj, M., Fisher, D.E., Kontaridis, M.I., Look, A.T., and Neel, B.G.
- Source
- Full text @ Dev. Cell
|
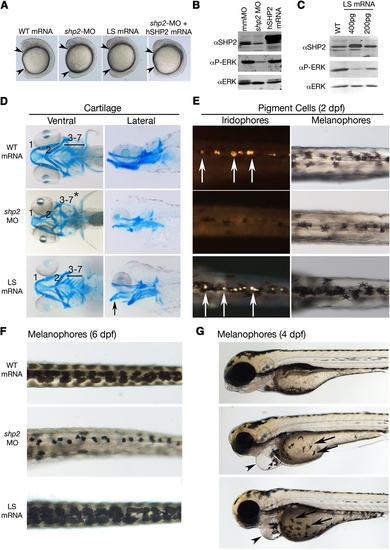
Effects of LEOPARD Syndrome Mutants Are Not Equivalent to shp2 Deficiency (A) Lateral views of 12 somite stage embryos injected with the indicated mRNAs or MOs. Arrowheads indicate distance between the head and tail. (B) Immunoblot showing lysates from embryos injected with the shp2 MO, compared with control (mmMO) or WT human PTPN11 (hSHP2) mRNA. Total Erk is indicated as a loading control. (C) Immunoblot showing dose-dependent dominant-negative effects on pErk levels in embryos injected with LSA462T mRNA compared with uninjected controls (WT). (D) Alcian blue stains of 6 dpf embryos injected with the indicated mRNAs or shp2 MO. Left panels, ventral view; right panels, lateral view. (E) Dorsal views of 2 dpf embryos, in dark (left) and bright (right) field, showing loss of iridophores (white arrows) and melanophores in shp2 MO-injected embryos. LS mRNA injection increases pigment cell numbers. (F) Dorsal views of melanophores at 6 dpf, showing pigment cells in WT mRNA-, MO-, and LS mRNA-injected embryos. (G) Lateral views of 4 dpf embryos. Note delayed migration of melanophores to the yolk in MO- and LS mRNA-injected embryos (black arrows), cardiac edema (arrowheads), and jaw defects. See Figure S1 for additional quantification and data. PHENOTYPE:
|
|
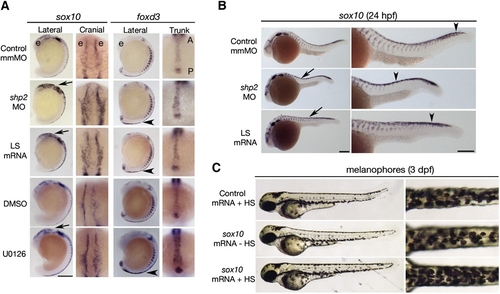
Phosphatase- and Erk-Dependent Role of Shp2 in Neural Crest Specification and Migration (A) Indicated views of in situ hybridizations with sox10 and foxd3 probes from control, MO-, LS mRNA-injected, DMSO-, and U0126-treated embryos (16–18 somites). The head is to the top in all views. Note increased sox10 expression in the cranial neural crest in MO- and LS mRNA-injected embryos (arrows). Cranial views of the same region indicated by the arrow show delayed neural crest migration. At the same stage, foxd3 expression is elevated in trunk neural crest (arrowheads indicate region of higher magnification views). WT embryos treated with U0126 (150 μM) at the tailbud stage show elevated sox10 and foxd3 expression and delayed migration in cranial and trunk neural crest. (B) Lateral views of 26 somite embryos showing increased sox10 expression in MO- and LS mRNA-injected embryos in the head and along the dorsal midline (arrows). Trunk neural crest migration, as indicated by the most posterior migrating cells (arrowheads), also is delayed. Right panels show higher magnifications. (C) Lateral (left) and dorsal (right) views of WT embryos injected with control (gfp) or heat shock-inducible sox10 mRNA. A 2 hr heat shock at the 16–18 somite stage causes increased pigmentation in sox10 mRNA-injected embryos at 3 dpf; see Figure S2 for quantification and data. Scale bars are 200 μm; position of the eye (e) and the anterior–posterior (A–P) position in the trunk views is indicated. EXPRESSION / LABELING:
PHENOTYPE:
|
|
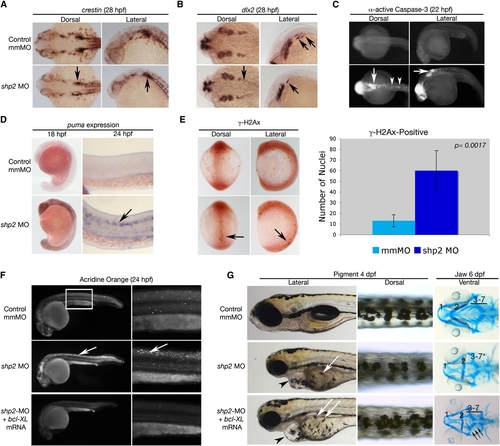
Loss of shp2 Causes Cell Death in Neural Tissue (A and B) Embryos (28 hpf) stained for the panneural crest marker crestin (A) or dlx2 (B). shp2 MO causes a severe loss of crestin- and dlx2-positive neural crest cells posterior to the otic placode (black arrows in [A] and [B]). (C) Views of 22 hpf embryos stained for α-activated-Caspase 3. Lack of shp2 causes apoptosis in the head and cells adjacent to the otic placode (arrows), extending down the dorsal region of the trunk (arrowheads). (D) Lateral views show increased puma expression in morphants at 18 hpf, particularly in the brain (left panels), which by 24 hpf becomes restricted to the neural tube (arrow in right panel). (E) Depletion of shp2 causes an increase in γ-H2Ax-positive nuclei in 12 somite stage embryos along the dorsal midline (quantified at right). (F) Lateral views (30x) of Acridine orange-stained embryos (24 hpf); right panels are higher magnification views (80x) of the trunk region (boxed). Cell death in the spinal cord (arrows) and brain is rescued by coexpressing zebrafish bcl-xl mRNA with shp2 MO. (G) Lateral (left) and Dorsal (right) views of 4 dpf embryos, showing that coexpression of zebrafish bcl-xl mRNA restores pigment cell numbers, but not their migration to the ventral stripe (white arrows). Right panels: Ventral views of 6 dpf embryos stained with Alcian blue, showing rescue of some cartilage elements (arrows). Error bars correspond to standard deviation of the mean. EXPRESSION / LABELING:
PHENOTYPE:
|
|
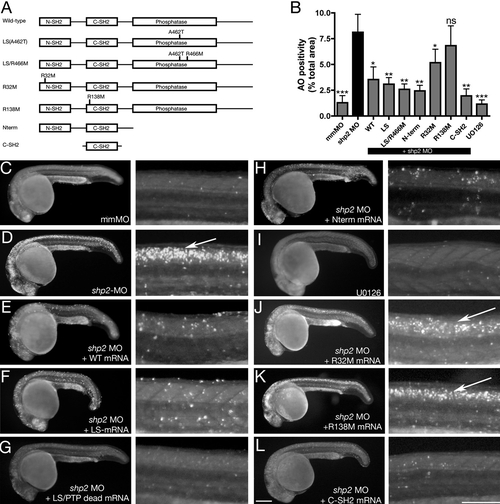
Antiapoptotic Function of Shp2 Is Mediated by Its SH2 Domains (A) Schematic of Shp2 mutants (also see Figure S1A). (B) Acridine orange uptake in the dorsal neural tube of zebrafish embryos injected with the indicated mRNAs. (C–L) Paired lateral views of 24 hpf embryos stained with Acridine orange; for each pair, the right panel shows a higher magnification image. (C) Control MO. (D) shp2 Ex3-MO causes cell death in the developing brain and spinal cord (arrow). Coexpression of WT shp2 (E), an LS mutant (A462T) (F), an A462T/R466M double mutant (G), or a truncation mutant that removes the PTP domain and C terminus (H), also blocks cell death. (I) WT embryos treated with U0126 do not mimic the morphant cell death phenotype. (J and K) Coexpression of shp2 mRNA with an N-SH2 point mutation (J) partially blocks cell death in the trunk; C-SH2 point mutations (K) fail to rescue. Coexpression of the C-SH2 domain alone rescues MO-induced cell death (L). Scale bars represent 200 μm. See Figures S1A and S3 for supporting data. Error bars correspond to standard deviation of the mean. PHENOTYPE:
|
|
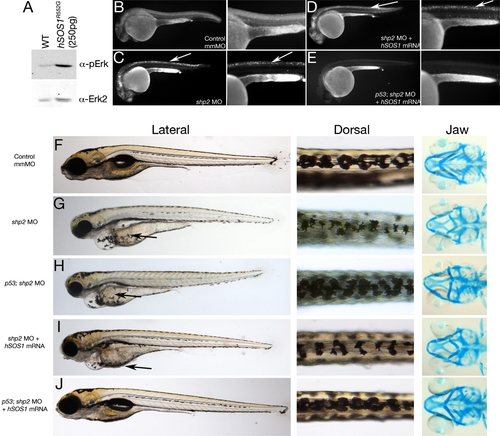
Restoring Both Shp2-Dependent Pathways in shp2 MO Embryos Rescues Neural Crest Phenotypes (A) Immunoblot showing effect of hSOS1R552G mRNA on Erk activation. (B–E) Lateral views of Acridine orange labeling in 24 hpf embryos. Right panels in each pair are higher magnification views. Cell death caused by shp2 MO (arrows in [C]) is not rescued by coexpression with hSOS1R552G mRNA (arrows in [D]) in WT embryos, but is rescued in p53 mutant embryos (E). (F–J) Lateral (left) and dorsal (middle) views of live 5 dpf embryos and ventral views (right) of 6 dpf embryos stained with Alcian blue. (G) Injection of shp2 MO causes severe jaw, heart, and pigmentation defects and delayed cell migration (arrow). (H) Injection of the shp2 MO into p53 mutant embryos partially rescues the jaw defects, but restores pigment cell numbers, although migration is still delayed (arrows in [G] and [H]). (I) Migration of pigment cells to the ventral stripe is rescued in shp2 MO embryos coinjected with hSOS1R552G mRNA (arrow), but pigment cell numbers are still reduced, particularly along the dorsal stripe (arrowheads), and embryos have smaller heads, heart edema, and severe craniofacial defects. (J) Coinjection of hSOS1R552G mRNA into p53 mutant embryos completely rescues neural crest phenotypes. |
|
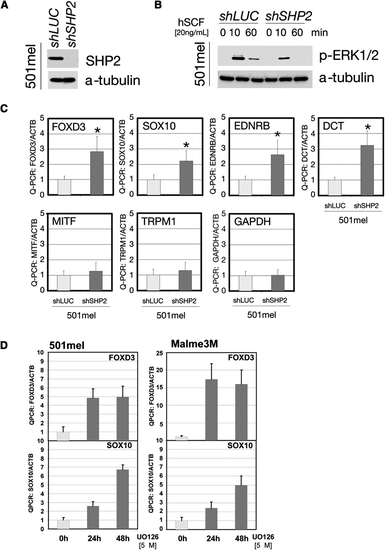
SHP2 Regulates Transcription Factors in Human Neural Crest-Derived Cells (A) Immunoblot of lysates from 501mel cells stably expressing SHP2-shRNA. (B) ERK activation in 501mel cells transduced with SHP2-shRNA#2 or shLUC lentivirus at the indicated times after stem cell factor stimulation (hSCF). (C) Levels of neural crest and melanocyte transcription factors in shRNA-transduced 501mel cells, determined by Q-PCR. SHP2 deficiency increases transcription of early progenitor (SOX10, FOXD3, DCT, and EDNRB), but not differentiation (MITF, TRPM1) markers. GAPDH is included as a control and all levels are expressed as ratios relative to β-actin (internal control). Asterisk: p < 0.05 by Student′s t test. (D) Levels of neural crest and melanocyte markers in 501mel and Melme3M cells after U0126 treatment, showing that the negative regulation of SOX10 and FOXD3 is ERK dependent. Error bars correspond to standard deviation of the mean. |
|
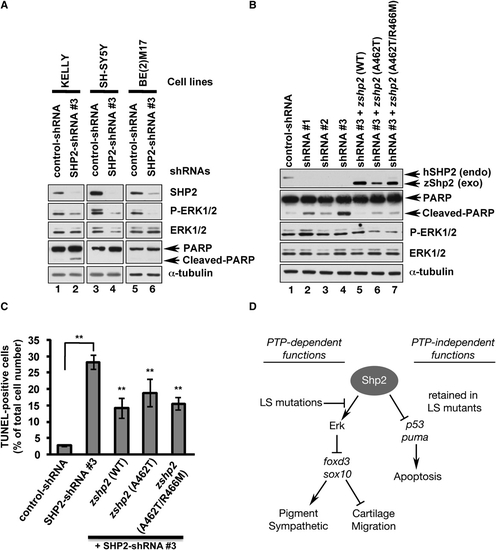
PTP- and ERK-Independent Function Of SHP2 Is Conserved in Neuroblastoma Cells (A) Immunoblot of lysates from neuroblastoma cell lines transduced with control shRNA or SHP2-shRNA#3. SHP2 and p-ERK1/2 levels are reduced by shRNA#3 in all cell lines (lanes 2, 4, 6), but only KELLY cells exhibit increased PARP cleavage (lane 2). (B) KELLY cells transduced with SHP2-shRNAs (lanes 1–3) have decreased SHP2 and ERK1/2 levels and increased PARP cleavage (compare lane 1 to lanes 2–4). PARP cleavage caused by shRNA#3 is rescued by cotransduction of wild-type zebrafish zshp2, PTP-impaired (A462T) or PTP-dead (A462T/R466M) zshp2. Exogenous WT zshp2 partially rescues p-ERK1/2 levels (while LS or LS/PTP-dead zshp2 have mild dominant-negative effects). (C) shRNA#3 induces cell death in ∼30% of the infected KELLY cells (TUNEL stain). Cotransduction with zebrafish constructs reduces the cell death caused by shRNA#3. **p < 0.001. (D) Model showing phosphatase-dependent and -independent functions of Shp2 during neural crest development. See text for details and Figure S4 for supporting data. Error bars correspond to standard deviation of the mean. |
|
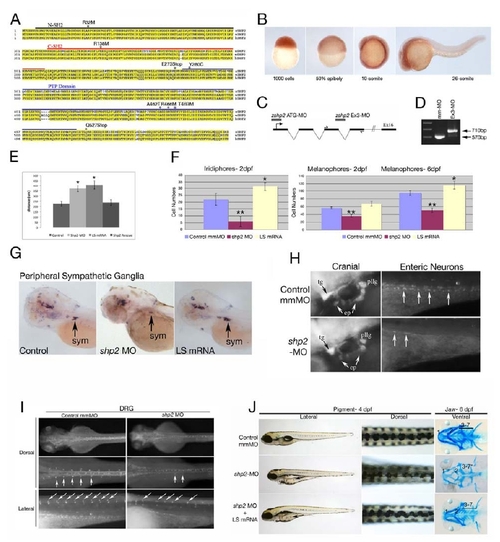
(A) Zebrafish Shp2 encodes a protein of 597 amino acids with 92% identity and 99% similarity to its murine and human orthologs. The N-SH2, C-SH2 and PTP domains are underlined black, red and blue, respectively. The locations of point mutations and truncations used in this study are indicated above the sequence. (B) Analysis of shp2 mRNA expression during embryogenesis by whole-mount in situ hybridization, showing ubiquitous maternal expression at blastula (1000-cell) and gastrulation stages. At segmentation stages, shp2 levels are highest in the developing CNS and spinal cord. (C) Schematic of shp2 genomic structure, showing the translation-blocking (ATG-MO) and splice-blocking (Ex3-MO) antisense morpholinos used in this study. The primers used to confirm interruption of splicing by Ex3-MO are indicated. (D) RT-PCR of embryos injected with Ex3-MO, showing that it prevents use of the normal shp2 exon 3 splice donor site; instead, a cryptic donor site 140 bp downstream in intron 4 is used (data not shown) and a larger PCR product results. (E) Quantification of gastrulation phenotypes represented in Figure 1A, as determined by measuring the distance from the tip of the head to the tailbud. Co-expression of human SHP2 mRNA rescues the shp2 MO gastrulation phenotypes, *p <0.005. (F) Quantification of the pigment cell defects associated with LS mRNA- and shp2 MO-injected embryos; n= 10 for all samples, *p <0.002, **p <0.0001. (G) Lateral views of peripheral sympathetic neurons (sym) detected by RNA in situ hybridization for tyrosine hydroxylase (th) at 4 dpf. Injection of the shp2 MO causes ~ 60% (p <0.002) loss of th-positive cells in the superior cervical ganglia (sym), whereas injection of LS mRNA causes a ~40% (p <0.003) increase in the number of these neurons (arrows). (H) Lateral view showing immunofluorescence labeling of sensory cranial ganglia and enteric neurons with α-Hu-C (HuC) at 4 dpf. shp2 MO-injected embryos have reduced numbers of trigeminal (tg), epibranchial (ep) and posterior lateral line (pllg) neurons and severe loss of enteric neurons (ent) neurons. (I) Dorsal root ganglia (DRG) neurons labeled with HuC at 4 dpf show loss and displacement in shp2 MO-injected embryos (arrows). (J) Co-injection of LS mRNA and shp2 MO results in LS-like NC phenotypes. Lateral (left) and dorsal (middle) views of live 4 dpf embryos, and ventral views (right) of 6 dpf embryos stained with Alcian blue. Injection of shp2 MO causes loss of pigment and jaw elements, as well as delayed migration to the ventral stripe (arrow). Co-injection of LS mRNA (A462T) with the shp2 MO (bottom) results in a LS-like pigment and jaw phenotypes in 70-80% of embryos (n=100), with delayed migration to the ventral stripe (compare with Figs. 1 D-G). Pigment numbers at 2 dpf: Control- 58.25 (SD= 2.6), shp2 MO- 35.75 (SD= 3.2), shp2 MO +LS mRNA= 66.0 (SD= 5.12). p <0.0001 for comparison of shp2 MO to shp2 MO + LS mRNA. Error bars correspond to standard deviation of the mean. |
Reprinted from Developmental Cell, 18(5), Stewart, R.A., Sanda, T., Widlund, H.R., Zhu, S., Swanson, K.D., Hurley, A.D., Bentires-Alj, M., Fisher, D.E., Kontaridis, M.I., Look, A.T., and Neel, B.G., Phosphatase-Dependent and -Independent Functions of Shp2 in Neural Crest Cells Underlie LEOPARD Syndrome Pathogenesis, 750-762, Copyright (2010) with permission from Elsevier. Full text @ Dev. Cell