- Title
-
A synaptic corollary discharge signal suppresses midbrain visual processing during saccade-like locomotion
- Authors
- Ali, M.A., Lischka, K., Preuss, S.J., Trivedi, C.A., Bollmann, J.H.
- Source
- Full text @ Nat. Commun.
|
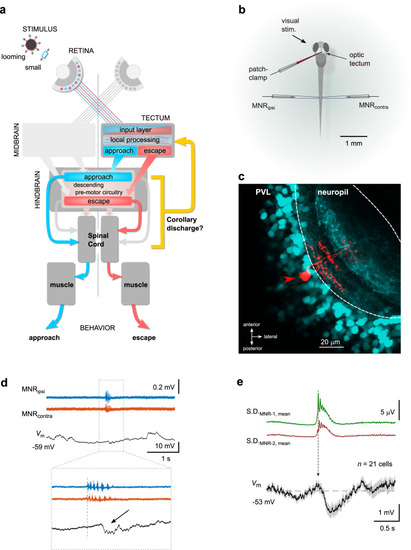
Patch-clamp recordings reveal a motor-related voltage signal in tectal neurons. |
|
During spontaneous swim bouts, tectal neurons receive motor-related phasic inhibition. |
|
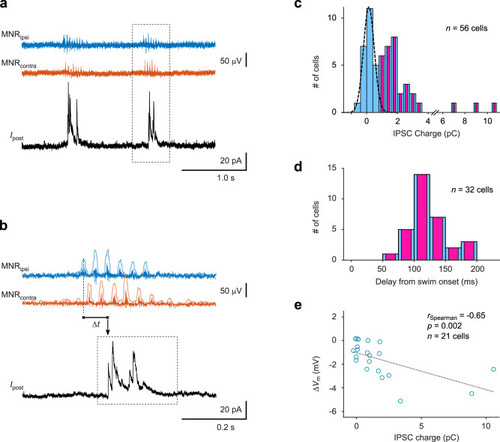
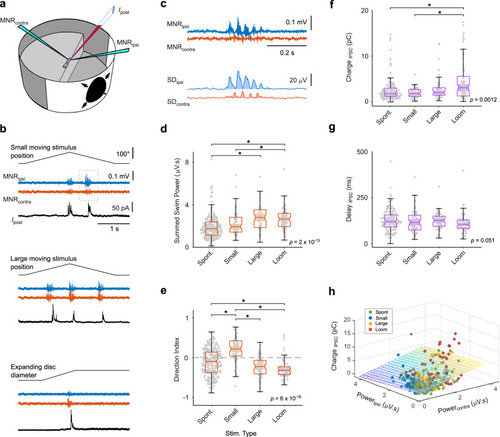
Fast inhibitory currents in tectal cells during visually driven, directed swimming. |
|
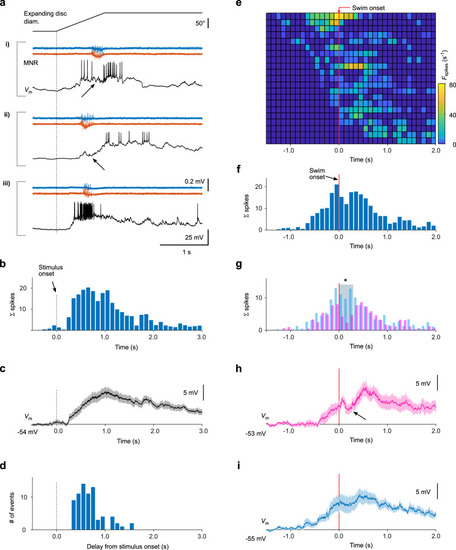
Swim-related inhibition transiently suppresses visually evoked spike output. |
|
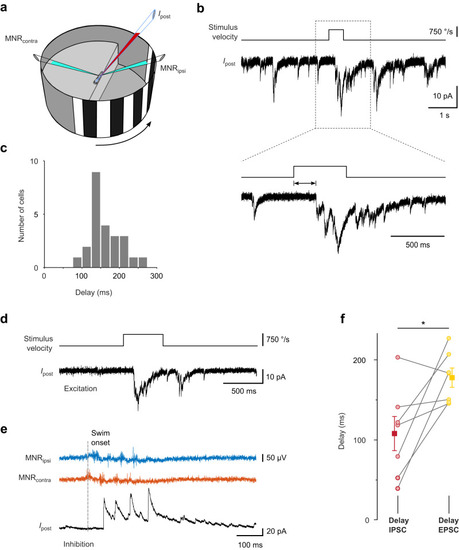
Delay of excitatory input evoked by fast whole-field motion stimuli. |
|
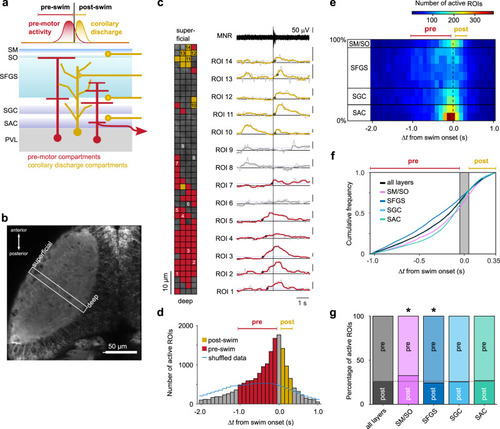
Spatial distribution of swim-related Ca2+ signals in the tectal neuropil. |
|
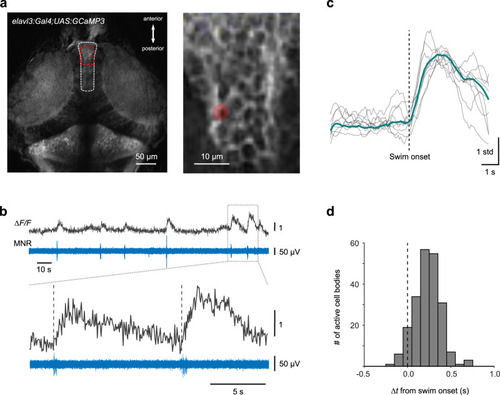
Temporal distribution of swim-related Ca2+ signals in the torus longitudinalis (TL). |
|
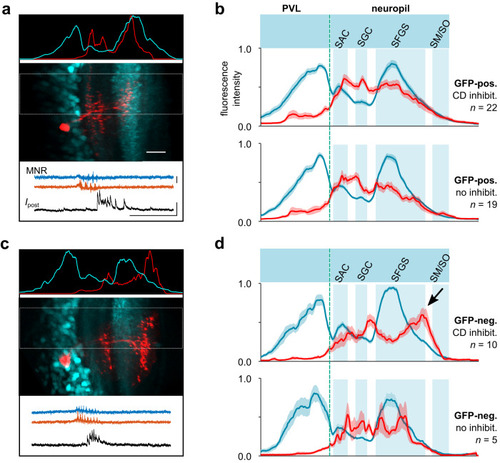
Dendritic profiles of tectal cells with CD synaptic inhibition. |
|
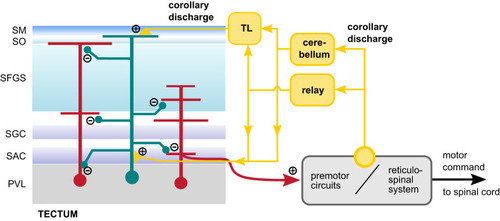
Putative mechanism of CD signaling in the visuomotor pathway. Hypothesized organization of inhibitory CD signaling in the tectum. Diverse cell types in the tectum (red cells) receive motor-related inhibitory inputs during swimming. The CD signal is initiated at unknown sites in premotor circuitry controlling tail and eye movements (yellow circle) and is relayed to the tectum, possibly via the cerebellum, or other relay nodes. Because post-swim Ca2+ signals cluster in the most superficial layer containing the |