- Title
-
Mutation of vsx genes in zebrafish highlights the robustness of the retinal specification network
- Authors
- Letelier, J., Buono, L., Almuedo-Castillo, M., Zang, J., Mounieres, C., González-Díaz, S., Polvillo, R., Sanabria-Reinoso, E., Corbacho, J., Sousa-Ortega, A., Diez Del Corral, R., Neuhauss, S.C.F., Martínez-Morales, J.R.
- Source
- Full text @ Elife
|
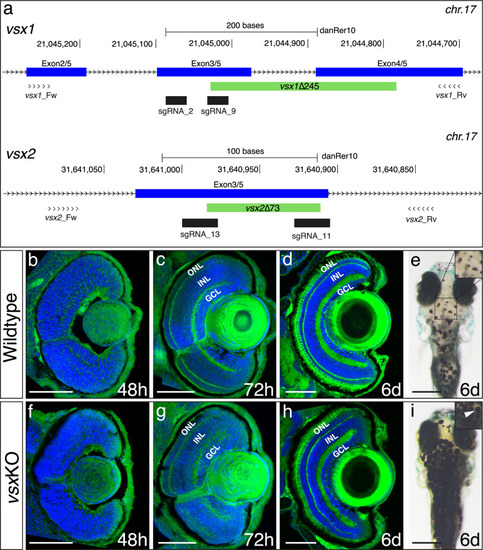
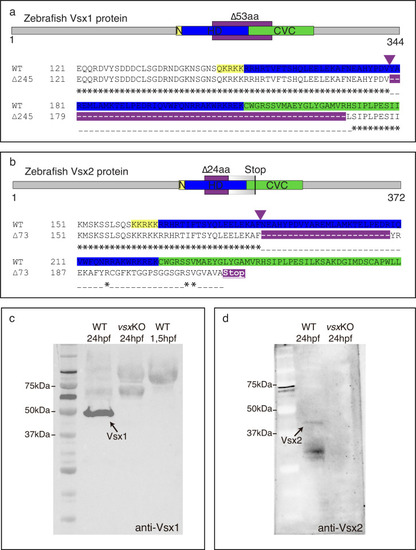
(a, b). Scheme of zebrafish Vsx1 and Vsx2 peptides and protein alignments showing the nuclear localization signal (N, yellow), homeodomain (HD, blue) and CVC (green) regions. The starting of the mutation induced by CRISPR/Cas9 is indicated by a purple arrowhead. (a). The in-frame 53 amino acid deleted region in the vsx1 mutant is depicted by a purple color in the upper scheme and in the bottom protein alignment. (b). The 24 amino acid deleted region in the vsx2 mutant is depicted by a purple color in the upper scheme followed by a graded grey box indicating the frame-shift in the sequence and the premature stop codon generated in the CVC domain. Important to note is that the deletion in vsx2 comprised conserved critical amino acids that have been identified as causative mutations both in mice (ocular retardation model) and microphthalmic patients (R200Q/P and R227W mutations). (c). Western blot representative image using an anti-Vsx1 antibody showing the WT Vsx1 protein (black arrow). Note that Vsx1 protein is not detected either in vsxKO at 24hpf (middle lane) or in early (1.5hpf) wildtype embryos (right lane). (d). Western blot representative image using an anti-Vsx2 antibody showing the WT Vsx2 protein at 24hpf (black arrow). No Vsx2 protein is detectable in vsxKO heads at 24hpf. |
|
(a, b). Scheme of zebrafish Vsx1 and Vsx2 peptides and protein alignments showing the nuclear localization signal (N, yellow), homeodomain (HD, blue) and CVC (green) regions. The starting of the mutation induced by CRISPR/Cas9 is indicated by a purple arrowhead. (a). The in-frame 53 amino acid deleted region in the vsx1 mutant is depicted by a purple color in the upper scheme and in the bottom protein alignment. (b). The 24 amino acid deleted region in the vsx2 mutant is depicted by a purple color in the upper scheme followed by a graded grey box indicating the frame-shift in the sequence and the premature stop codon generated in the CVC domain. Important to note is that the deletion in vsx2 comprised conserved critical amino acids that have been identified as causative mutations both in mice (ocular retardation model) and microphthalmic patients (R200Q/P and R227W mutations). (c). Western blot representative image using an anti-Vsx1 antibody showing the WT Vsx1 protein (black arrow). Note that Vsx1 protein is not detected either in vsxKO at 24hpf (middle lane) or in early (1.5hpf) wildtype embryos (right lane). (d). Western blot representative image using an anti-Vsx2 antibody showing the WT Vsx2 protein at 24hpf (black arrow). No Vsx2 protein is detectable in vsxKO heads at 24hpf. |
|
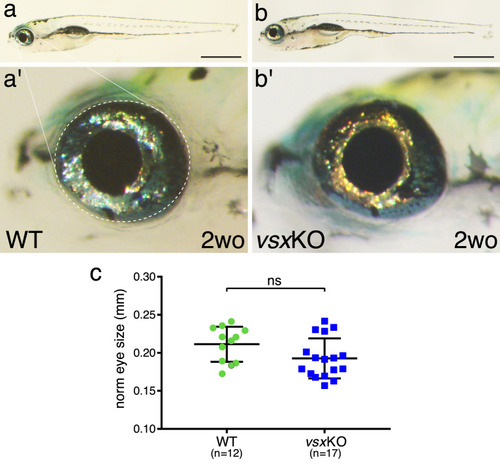
(a, b). Lateral view of a 2-week-old wildtype (a) and vsxKO (b) fish. Note that vsxKO juvenile fish show no obvious morphological differences with wildtype siblings. (a', b'). High-magnification images from wildtype ( |
|
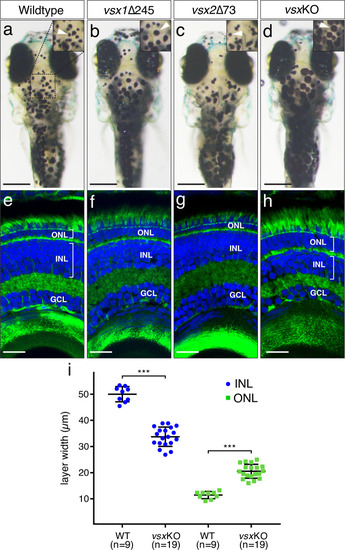
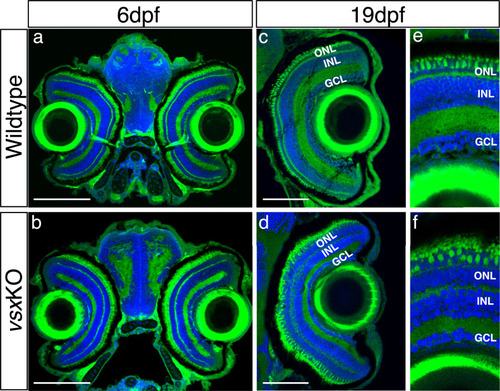
(a-d). Dorsal view of wildtype (a), vsx1∆245 (b), vsx2∆73 (c), and vsxKO (d) animals at 6dpf. vsx1∆245 (b) and vsxKO (d) fish have problems sensing background light and appear darker than wildtype (a) and vsx2∆73 (c) larvae, as melanosomes are broadly distributed within melanophores (white arrowheads). (e-h). Histological sections of wildtype (e), vsx1∆245 (f), vsx2∆73 (g), and vsxKO (h) eyes at 6dpf. In the central retina of vsxKO double mutants (h), the INL width is reduced and the ONL thickness is expanded compared to wildtype (e), vsx1∆245 (f), or vsx2∆73 (g) samples. DAPI and phalloidin coupled to Alexa488 were used as nuclear and cytoskeletal (actin) markers, respectively. (i). Quantification of INL (blue circles) and ONL (green squares) width in central retinas at 6dpf using histological sections as in (e-h) panels. Significant reduction and expansion of the INL and ONL, respectively, was observed in vsxKO retinas when compared to wildtype (***p<0.0001) using an unpaired t-test. Data are shown as mean ± SD. ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer, µm: micrometres. Scale bar in (a-d): 500µm, scale bar in (e-h): 20µm. |
|
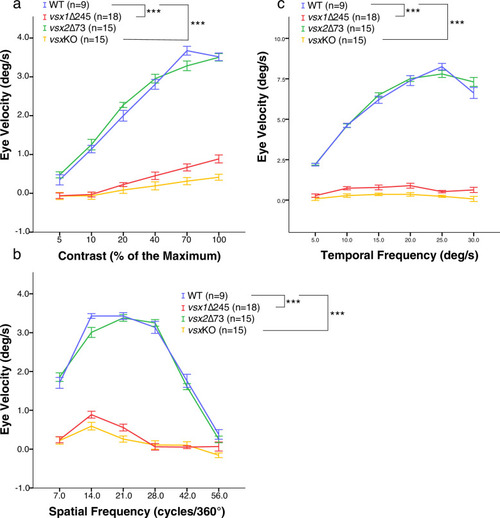
(a-c). OKR was recorded in wildtype (blue), vsx1∆245 (red), vsx2∆73 (green), and vsxKO double mutants (yellow) at 5dpf in response to different contrast (a), spatial frequency (b) and angular velocity (c). OKR is significantly reduced in vsx1∆245 and vsxKO double mutant larvae in comparison to WT siblings (***p<0.001) using a one-way ANOVA. Note that no differences in OKR were observed between WT and vsx2∆73 single mutant or between vsx1∆245 and vsxKO mutants (p>0.05). In (a-c), vsx1∆245 (red tracks) represents both vsx1∆245-/- and vsx1∆245-/-; vsx2∆73+/-genotypes, while vsx2∆73 (green tracks) represents both vsx2∆73-/- and vsx1∆245+/-; vsx2∆73-/- genotypes. In all panels, data is shown as mean ± SEM. deg: degrees, s: seconds. |
|
(a-c). OKR was recorded in wildtype (blue), vsx1∆245 (red), vsx2∆73 (green), and vsxKO double mutants (yellow) at 5dpf in response to different contrast (a), spatial frequency (b) and angular velocity (c). OKR is significantly reduced in vsx1∆245 and vsxKO double mutant larvae in comparison to WT siblings (***p<0.001) using a one-way ANOVA. Note that no differences in OKR were observed between WT and vsx2∆73 single mutant or between vsx1∆245 and vsxKO mutants (p>0.05). In (a-c), vsx1∆245 (red tracks) represents both vsx1∆245-/- and vsx1∆245-/-; vsx2∆73+/-genotypes, while vsx2∆73 (green tracks) represents both vsx2∆73-/- and vsx1∆245+/-; vsx2∆73-/- genotypes. In all panels, data is shown as mean ± SEM. deg: degrees, s: seconds. |
|
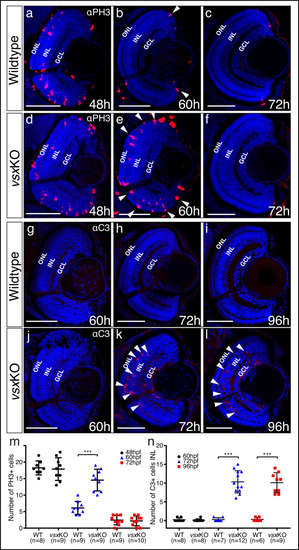
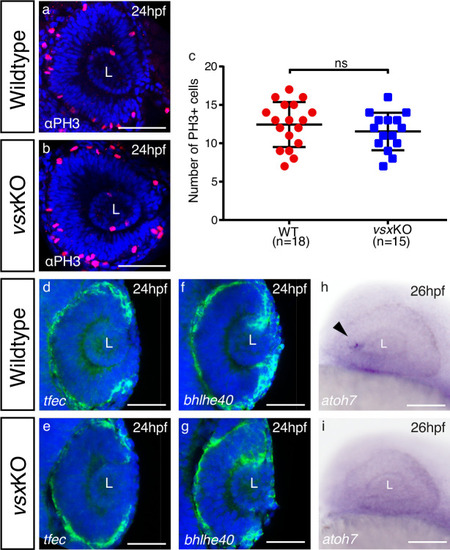
(a, b). Phospho‐histone H3 (PH3) antibody staining was used to evaluate proliferation in WT (a) and vsxKO (b) retinas at 24hpf. (c). Quantification of PH3 positive cells in WT and vsxKO retinas at 24hpf. No significant differences (p>0.05) were detected at that stage between both samples using an unpaired t-test. Data is shown as mean ± SD. (d-g). Confocal representative images of RPE markers tfec (d, e) and bhlhe40 (f, g) by fluorescent in situ hybridization at 24hpf. No major changes in the expression of both genes are detected in vsxKO embryos (e, g) compared to WT samples (d, f), n≥6. (h, i). Lateral view (anterior to the left) from 26hpf WT (h) and vsxKO (i) heads showing the expression of atoh7 gene by colorimetric in situ hybridization. The onset of atoh7 expression is delayed in mutant retinas compared to WT animals. Note an atoh7 positive cell in WT eye at that stage (black arrowhead) n≥12. L: lens, ns: not significant. Scale bar: 50µm . |
|
(a, b). Phospho‐histone H3 (PH3) antibody staining was used to evaluate proliferation in WT (a) and vsxKO (b) retinas at 24hpf. (c). Quantification of PH3 positive cells in WT and vsxKO retinas at 24hpf. No significant differences (p>0.05) were detected at that stage between both samples using an unpaired t-test. Data is shown as mean ± SD. (d-g). Confocal representative images of RPE markers tfec (d, e) and bhlhe40 (f, g) by fluorescent in situ hybridization at 24hpf. No major changes in the expression of both genes are detected in vsxKO embryos (e, g) compared to WT samples (d, f), n≥6. (h, i). Lateral view (anterior to the left) from 26hpf WT (h) and vsxKO (i) heads showing the expression of atoh7 gene by colorimetric in situ hybridization. The onset of atoh7 expression is delayed in mutant retinas compared to WT animals. Note an atoh7 positive cell in WT eye at that stage (black arrowhead) n≥12. L: lens, ns: not significant. Scale bar: 50µm . |
|
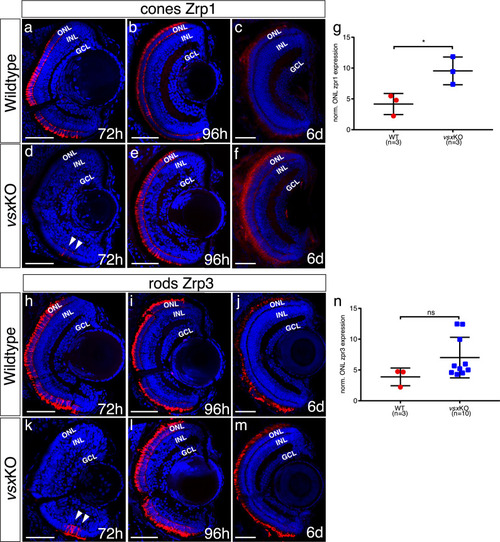
(a-f), (h-m). Cryosections of wildtype (a-c, h-j) and vsxKO retinas (d-f, k-m) stained with photoreceptor specific antibodies and DAPI as a nuclear marker. Cones and rods were visualized by using the Zpr1 (a-f) and Zpr3 (h-m) antibodies, respectively. Delayed cone and rod differentiation is observed in vsxKO mutants (d, k) compared to wildtype (a, h) samples at 72hpf (white arrowheads in d, k). At 96hpf, both markers are expressed in similar levels in wildtype (b, i) and double mutant retinas (e, l), but at 6dpf there is a significant increase of Zpr1 fluorescent intensity in vsxKO (f) compared to WT retinas (c) using an unpaired t-test (*p<0.05). No major differences were observed in rod stain intensity between WT (j) and vsxKO (m) at 6dpf (p>0.05). (g, n). Quantification of cones (g) and rods (n) label intensity between WT and vsxKO retinas were performed using Fiji software. Fluorescent measurements in the ONL were normalized by background signal from the INL. Data is shown as mean ± SD. For all conditions tested (except for c, f, j) n≥10. ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer, h: hours post-fertilization, d: days post-fertilization, ns: not significant. |
|
(a-f), (h-m). Cryosections of wildtype (a-c, h-j) and vsxKO retinas (d-f, k-m) stained with photoreceptor specific antibodies and DAPI as a nuclear marker. Cones and rods were visualized by using the Zpr1 (a-f) and Zpr3 (h-m) antibodies, respectively. Delayed cone and rod differentiation is observed in vsxKO mutants (d, k) compared to wildtype (a, h) samples at 72hpf (white arrowheads in d, k). At 96hpf, both markers are expressed in similar levels in wildtype (b, i) and double mutant retinas (e, l), but at 6dpf there is a significant increase of Zpr1 fluorescent intensity in vsxKO (f) compared to WT retinas (c) using an unpaired t-test (*p<0.05). No major differences were observed in rod stain intensity between WT (j) and vsxKO (m) at 6dpf (p>0.05). (g, n). Quantification of cones (g) and rods (n) label intensity between WT and vsxKO retinas were performed using Fiji software. Fluorescent measurements in the ONL were normalized by background signal from the INL. Data is shown as mean ± SD. For all conditions tested (except for c, f, j) n≥10. ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer, h: hours post-fertilization, d: days post-fertilization, ns: not significant. |
|
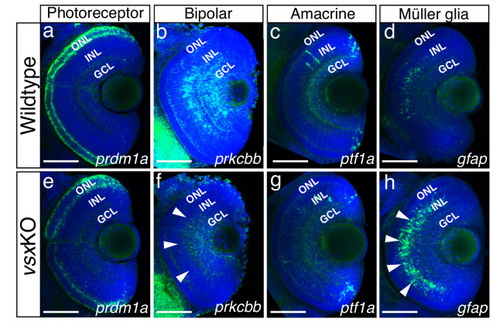
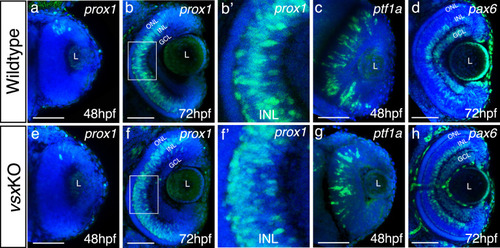
(a-h). Confocal representative images of genes expressed in the INL by fluorescent in situ hybridization at different stages. At 48hpf, no obvious change in the expression of prox1 was detected between WT (a) and vsxKO (e) retinas. At 72hpf, the distribution of prox1 expression in the INL is affected in vsxKO (f and inset f’) compared to WT retinas (b and inset |
|
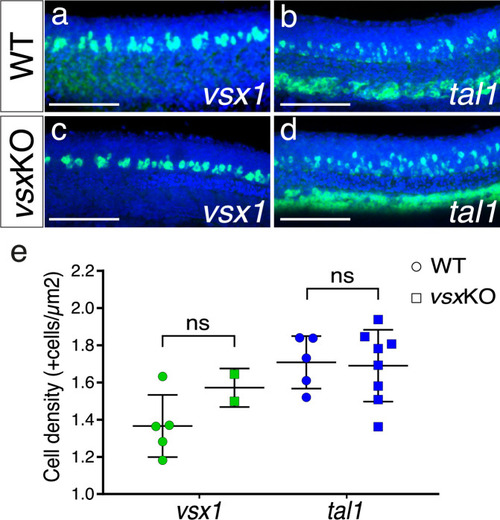
(a-d). Fluorescent in situ hybridization lateral images from 24hpf wildtype (a, b) and vsxKO (c, d) mutants using vsx1 (a, c) and tal1 (b, d) probes to visualize V2a and V2b trunk interneurons, respectively. (e). The density of vsx1 (V2a) and tal1 (V2b) positive neurons was measured in wildtype (n=5 for vsx1 and n=5 for tal1) and vsxKO (n=2 for vsx1 and n=7 for tal1) mutant trunks at 24hpf. Using an unpaired t-test, no significant changes in the number of V2a and V2b neurons was observed between wildtype and vsxKO mutant animals at this stage (p>0.05). Data is shown as mean ± SD. ns: not significant. Scale bar in (a-d): 100 µm. |
|
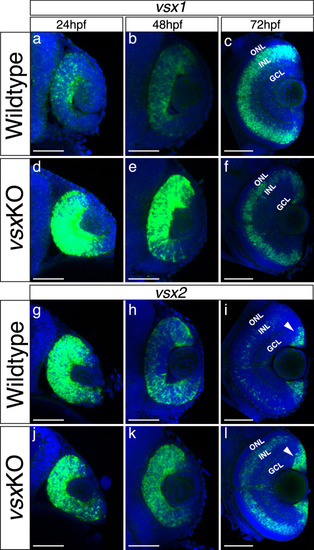
(a-l). Fluorescent in situ hybridization for vsx1 (a-f) and vsx2 (g-l) genes over retina formation in wildtype (a-c, g-i) and vsxKO (d-f) animals (j-l). In wildtype samples, vsx2 is strongly expressed than vsx1 in retinal precursors at earlier developmental stages (a, g) and the opposite effect is observed in INL bipolar cells at later stages (c, i). We found an overexpression of vsx1 at 24hpf (b) and 48hpf (e) and a reduction in the INL expression at 72hpf (c, f) in vsx mutant retinas compared to wildtype. For vsx2, no major differences between wildtype and mutant retinas were detected at 24hpf (g, j), but at 48 and 72hpf we found an increment of vsx2 in the retina and INL, respectively ( |
|
(a-l). Fluorescent in situ hybridization for vsx1 (a-f) and vsx2 (g-l) genes over retina formation in wildtype (a-c, g-i) and vsxKO (d-f) animals (j-l). In wildtype samples, vsx2 is strongly expressed than vsx1 in retinal precursors at earlier developmental stages (a, g) and the opposite effect is observed in INL bipolar cells at later stages (c, i). We found an overexpression of vsx1 at 24hpf (b) and 48hpf (e) and a reduction in the INL expression at 72hpf (c, f) in vsx mutant retinas compared to wildtype. For vsx2, no major differences between wildtype and mutant retinas were detected at 24hpf (g, j), but at 48 and 72hpf we found an increment of vsx2 in the retina and INL, respectively (h, k, i, l). Also, at 72hpf, there is a stronger expression of vsx2 in the ciliary marginal zone (CMZ) compared to WT. hpf: hours post fertilization, ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. Scale bar in (a-l): 50 µm. |
|
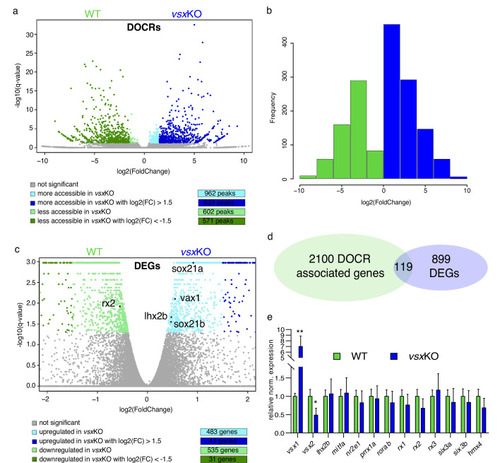
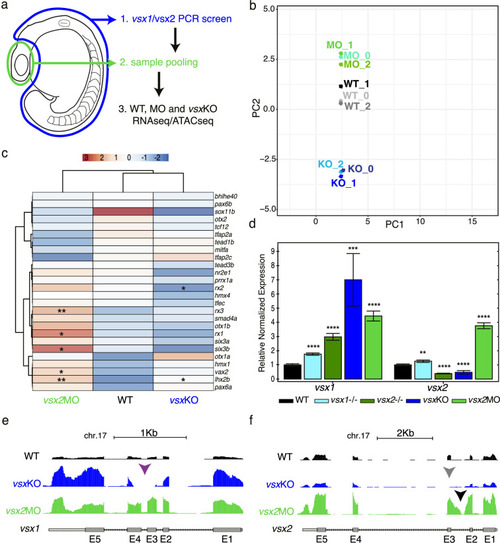
(a) Experimental design. Zebrafish heads including both eyes were mechanically dissected at 18hpf from WT, vsxKO and vsx2MO samples. The trunk and tail (highlighted in blue) were used to extract DNA and genotyping of WT and vsxKO embryos. After genotyping, samples from individual embryos previously dissected were pooled and used for ATAC-seq and/or RNA-seq protocols. (b). RNA-seq PCA evaluation revealing the transcriptomic divergence between WT (black/grey), vsxKO (blue) and vsx2MO (green) samples. (c). Heatmap chart showing gene expression differences between WT, vsxKO and vsx2MO samples of the main retinal transcriptional regulators (*p<0.05; p**<0.01). (d). vsx1 and vsx2 expression levels analysed by real time PCR (n=3) in WT, vsx1∆245, vsx2∆73, vsxKO, and vsx2MO samples. All statistical comparisons are relative to the WT animals using one-way ANOVA (**p<0.01; ***p<0.001; ****p<0.0001; no label = no significant differences). Data is shown as mean ± SD. (e). Graphical visualization of RNA-seq tracks showing vsx1 gene expression in WT, vsxKO, and vsx2MO eyes. In vsxKO samples (blue track), the deletion generated by CRISPR/Cas9 is noticeable due to the lack of reads in part of the third and fourth exons (purple arrowhead). (f). RNA-seq tracks showing vsx2 expression in WT, vsxKO, and vsx2MO samples. In vsxKO samples (blue track), the deletion is evident by the lack of reads in the third exon (grey arrowhead). In vsx2 morphants (green track), a retention of the second intron due to the action of the morpholino can be appreciated. |
|
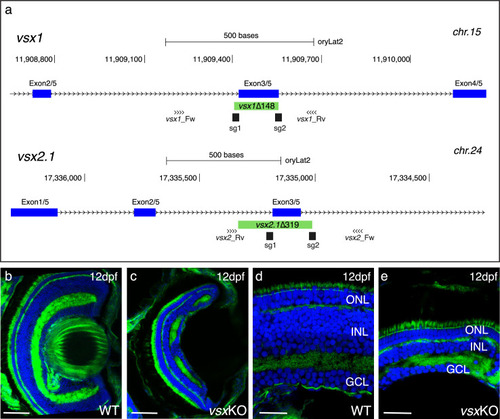
(a) CRISPR/Cas9 was used to eliminate (green box) the DBD from vsx1 (top) and vsx2.1 (bottom) TFs in medaka. Blue boxes represent exons, black boxes the location of sgRNAs used and primers for screening are depicted as opposing arrowheads. (b-e). Histological sections from WT ( |
|
(a-f). Visual system histological sections stained with nuclear marker DAPI and phalloidin-Alexa488 for actin filaments from wildtype (a, c and e, n=5 for both stages) and vsxKO (b, d and |