- Title
-
Live imaging and conditional disruption of native PCP activity using endogenously tagged zebrafish sfGFP-Vangl2
- Authors
- Jussila, M., Boswell, C.W., Griffiths, N.W., Pumputis, P.G., Ciruna, B.
- Source
- Full text @ Nat. Commun.
|
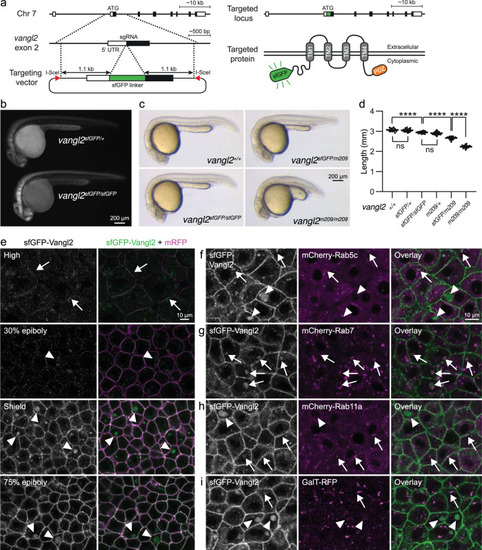
sfGFP-Vangl2 is functional and dynamically localized to membranes during early zebrafish embryogenesis.
a A schematic illustration of the sfGFP-Vangl2 knock-in targeting strategy. b Wide-field fluorescence images demonstrating sfGFP-Vangl2 expression in vangl2sfGFP/+ and vangl2sfGFP/sfGFP embryos at 28 h post-fertilization (hpf). c Lateral view of wild-type, vangl2sfGFP/sfGFP, vangl2sfGFP/m209 and vangl2m209/m209 embryos at 24 hpf. d Embryo length quantification at 24 hpf. Data shows representative experiments combined from independent crosses that were repeated 2-4 times. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparisons test (****P < 0.0001). Wild-type n = 14, vangl2sfGFP/+ n = 22, vangl2sfGFP/sfGFP n = 20, vangl2m209/+ n = 82, vangl2sfGFP/m209 n = 72, vangl2m209 n = 63. Horizontal line labels mean, and dots indicate individual embryo measurements. Source data are provided as a Source Data file. e Live confocal images of representative vangl2sfGFP/sfGFP (hereafter referred as vangl2sfGFP) embryos at blastula through early gastrula stages, as indicated (n = 6 for each stage). Ectodermal cells were imaged in gastrulating embryos. Embryos were injected with mRNA coding for a membrane-localized monomeric RFP (mRFP) reporter. All sfGFP images were acquired using identical settings. Arrows point at membrane-localized Vangl2 at high stage and arrowheads point at cytoplasmic Vangl2 puncta. Live confocal images of representative shield staged vangl2sfGFP embryos injected with mRNA coding for mCherry-Rab5c (f), mCherry-Rab7 (g), mCherry-Rab11a (h) or GalT-RFP (i) reporter constructs (n = 4 for each reporter). Arrows point at cytoplasmic Vangl2 puncta in close proximity to respective reporters, and arrowheads point at isolated Vangl2 puncta. |
|
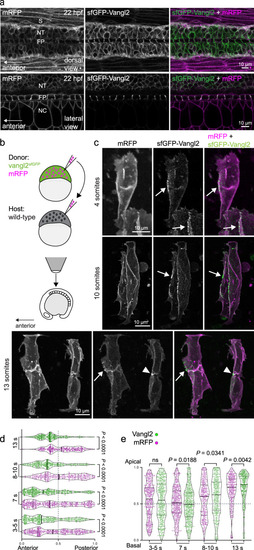
Vangl2 is enriched in a planar polarized manner on anterior neuroepithelial membranes.
a Live confocal images comparing sfGFP-Vangl2 and membrane-RFP localization at 22 h post fertilization (hpf) in dorsal (top) and lateral (bottom) orientations. Anterior is to the left in all images. Strong Vangl2 expression is observed on cell membranes in the neural tube (NT) including the floor plate (FP), while only weak expression is observed in somites (S) and notochord (NC). A representative image from n = 3 embryos shown. b A schematic illustrating transplantation of membrane-RFP-labelled vangl2sfGPP donor cells into WT (wild-type) host embryos, at sphere stage. Chimeric embryos were imaged dorsally, at positions between the 2nd and 8th somite. c Representative confocal images of membrane-RFP and sfGFP-Vangl2 localization in the developing spinal cord of chimeric embryos at 4-somite, 10-somite and 13-somite stages of development, as quantified in (d, e). Maximum intensity projections are shown. Arrows point to Vangl2 enrichment on anterior neuroepithelial cell membranes, and arrowheads to anterior apical membranes at the neural midline. Anterior is to the left in all images. Distribution of the brightest sfGFP-Vangl2 and membrane-RFP spots along anterior-posterior (d) and apical-basal (e) axes of vangl2sfGFP neuroepithelial cells, quantified at four consecutive stages of neural tube morphogenesis. Data from multiple transplanted vangl2sfGFP cells was pooled (3-5 somites, n = 7; 7 somites, n = 7; 8-10 somites, n = 6; 13 somites, n = 5) and axial lengths normalized to 1. Details of the quantification can be found in the Methods section. Statistical analysis was performed using a two-tailed Mann-Whitney test. Thicker line shows median, and thinner lines first and third quartile. Source data are provided as a Source Data file. EXPRESSION / LABELING:
|
|
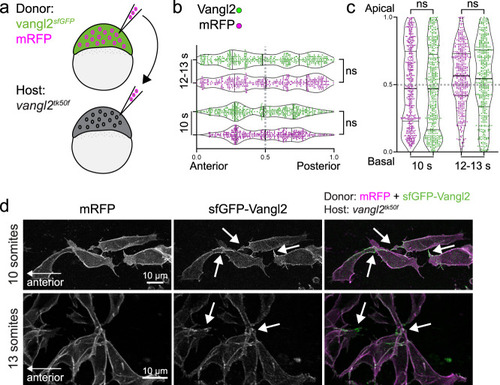
Planar polarized enrichment of Vangl2 is controlled by cell non-autonomous PCP signalling.
a A schematic illustrating transplantation of membrane-RFP-labelled vangl2sfGFP donor cells into vangl2tk50f loss-of-function host embryos, at sphere stage. b, c Distribution of the brightest sfGFP-Vangl2 and membrane-RFP spots along anterior-posterior (g) and apical-basal (h) axes of vangl2sfGFP neuroepithelial cells transplanted into a vangl2tk50f mutant host, quantified at two consecutive stages of neural tube morphogenesis. Data from multiple transplanted vangl2sfGFP cells was pooled (10 somites, n = 7; 12-13 somites, n = 9). Statistical analysis was performed using a two-tailed Mann-Whitney test. Thicker line shows median, and thinner lines first and third quartile. Source data are provided as a Source Data file. d Representative confocal images of membrane-RFP and sfGFP-Vangl2 localization in vangl2sfGFP neuroepithelial cells within vangl2tk50f mutant hosts, at 10-somite and 13-somite stages of development, as quantified in b, c. Maximum intensity projections are shown. Arrows point at Vangl2 enrichment on cell protrusions. Anterior is to the left in all images. |
|
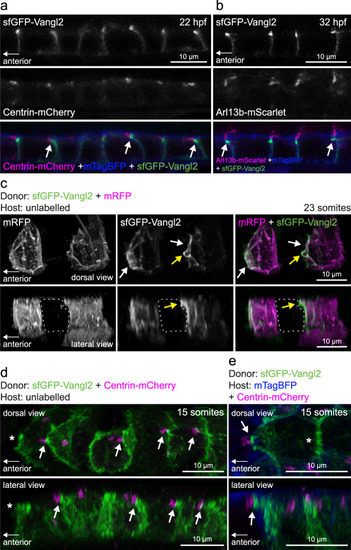
Vangl2-positive anterior membrane closely associates with planar polarized basal bodies of floor plate cells.
a Live confocal images of sfGFP-Vangl2 localization in relation to basal body (Centrin-mCherry) and membrane (mTagBFP-CAAX) reporters within floorplate cells of a vangl2sfGPP embryo at 22hpf. Lateral view, anterior to the left. Arrows point at sfGFP-Vangl2 enriched membranes extending apically towards anterior neighbouring cells. A representative image from n = 3 embryos shown. b Live confocal images of sfGFP-Vangl2 localization in relation to cilia axoneme (Arl13b-mScarlet) and membrane (mTagBFP-CAAX) reporters within floorplate cells of a vangl2sfGPP embryo at 32hpf. Lateral view, anterior is to the left. Arrows point at sfGFP-Vangl2 enriched membranes extending apically towards anterior neighbouring cells. A representative image from n = 5 embryos shown. c Live confocal image comparing sfGFP-Vangl2 and membrane-RFP localization in floorplate cells of a 23-somite staged chimeric embryo (mRFP mRNA-injected vangl2sfGFP cells transplanted into WT hosts). Maximum intensity projections, dorsal (top) and lateral (bottom) views are shown, anterior is to the left. White arrows point at Vangl2 enrichment on anterior cell membranes. Yellow arrows point at Vangl2 enriched membrane protruding from the anterior apical surface. Dashed line highlights an unlabelled host floorplate cell between two donor cells. A representative image from n = 16 cells shown. d Live confocal image comparing sfGFP-Vangl2 and Centrin-mCherry localization in floorplate cells of a 15-somite staged chimeric embryo (Centrin-mCherry mRNA-injected vangl2sfGFP cells transplanted into WT hosts). Maximum intensity projections, dorsal (top) and lateral (bottom) views are shown, anterior to the left. Arrows point at Vangl2 on the anterior membrane that is closely associated with a basal body docked on the posterior membrane. Asterisk indicates anterior unlabelled host cell that is not Centrin-mCherry positive. A representative image from n = 18 cells shown. e Live confocal image comparing sfGFP-Vangl2 localization in relation to basal body (Centrin-mCherry) and membrane (mTagBFP) reporters in neighbouring floorplate cells of a 15-somite staged chimeric embryo (vangl2sfGFP cells transplanted into WT hosts injected with Centrin-mCherry and mTagBFP-CAAX mRNA). Maximum intensity projections, dorsal (top) and lateral (bottom) views are shown, anterior to the left. Arrow points at Vangl2 on the anterior membrane of a donor cell that is closely associated with a basal body docked on the posterior membrane of a host cell. Asterisk indicates lack of Centrin-mCherry labelled basal body in the donor cell. A representative image from n = 17 cells shown. EXPRESSION / LABELING:
|
|
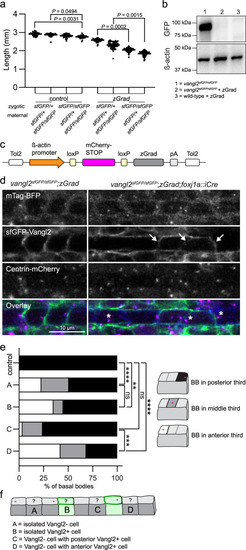
Conditional degradation of sfGFP-Vangl2 reveals a cell non-autonomous requirement for Vangl2 in maintaining basal body polarity.
a Quantification of embryo lengths for zGrad mRNA-injected and control embryos at 24 hpf. Maternal and zygotic genotypes are as indicated. Data is pooled from three independent experiments. Control n from left to right: n = 53, n = 62, n = 61, n = 36. zGrad n from left to right: n = 55, n = 51, n = 53, n = 54. Horizontal line labels mean, and dots indicate individual embryo measurements. Statistical analysis was performed using Kruskal-Wallis test with Dunn’s multiple comparisons test. Source data are provided as a Source Data file. b A representative Western blot detecting sfGFP-Vangl2 protein levels using a GFP antibody in uninjected vangl2 sfGFP/sfGFP embryos and vangl2sfGFP/sfGFP or wild-type embryos injected with 50 pg of zGrad mRNA. β-acting is used as a loading control. N = 3; 50 uninjected vangl2 sfGFP/sfGFP, 50 zGrad injected wild-type, and 70 zGrad injected vangl2 sfGFP/sfGFPembryos were lysed per sample. c A schematic of the conditional zGrad transgene. d Live confocal images of sfGFP-Vangl2 expression in relation to basal body (Centrin-mCherry) and membrane (mTagBFP) reporters within floorplate cells of control vangl2 sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad) embryos (left) and experimental vangl2 sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad);Tg(foxj1a::iCre) embryos (right) at 48 hpf. Dorsal view, anterior to the left. Arrows indicate residual sfGFP-Vangl2 expression in a subset of floorplate cells following zGrad expression. Asterisks indicate mispolarized basal bodies following zGrad expression. e Quantification of basal body localization within the posterior, medial or anterior thirds of floor plate cells for control and vangl2 sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad);Tg(foxj1a::cre) embryos described in b. Cells were categorized as A-D based on the presence or absence of sfGFP-Vangl2 signal within both the cell and its immediate neighbours, as illustrated in f. Control, n = 164; A, n = 256; B, n = 20; C, n = 29; D, n = 19. Statistical analysis was performed using Kruskal-Wallis test with Dunn’s multiple comparisons test. Source data are provided as a Source Data file. f A schematic illustrating cell categories quantified in e. Grey colour indicates cells that have lost sfGFP-Vangl2 signal; green colour indicates residual sfGFP-Vangl2 expression. PHENOTYPE:
|
|
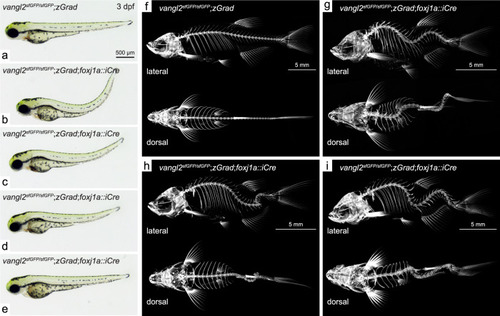
Degradation of sfGFP-Vangl2 in foxj1a-positive cell lineages causes embryonic axial curvature and postembryonic idiopathic-like scoliosis.
Overview of body axis phenotypes observed in control (a) and vangl2sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad);Tg(foxj1a::iCre) embryos (b–e) at 3 dpf. Lateral and dorsal projections of 3-dimensional micro-CT scans of 2 month old control (f) and vangl2sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad);Tg(foxj1a::iCre) fish (g–i). PHENOTYPE:
|
|
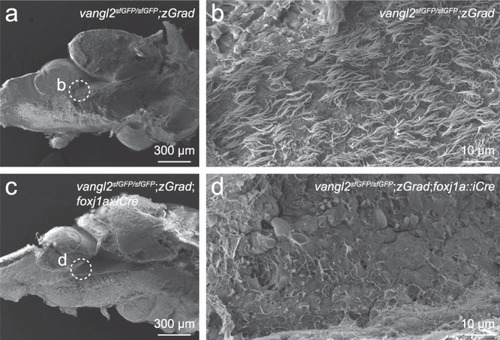
FoxJ1a-lineage specific degradation of sfGFP-Vangl2 results in ependymal cell cilia defects.
Scanning electron micrographs of the rhombencephalic ventricle in brains dissected from 7-8 month old vangl2sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad) control (a, b; n = 8) and vangl2sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad);Tg(foxj1a::iCre) mutant (c, d; n = 8) zebrafish. Dashed circles in a, c indicate the area of high magnification images in b, d. PHENOTYPE:
|
|
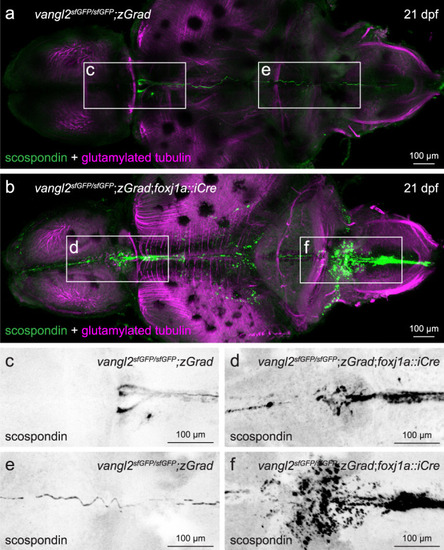
FoxJ1a-lineage specific degradation of sfGFP-Vangl2 results in ectopic scospondin accumulation and disrupted Reissner fiber formation.
a, b Representative maximum intensity Z-stack projections of confocal micrographs, acquired through dorsally-oriented whole mount brains that were dissected from 21dpf vangl2sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad) control (a; n = 4) and vangl2sfGFP/sfGFP;Tg(βactin2::loxP-mCherry-STOP-loxP-zGrad);Tg(foxj1a::iCre) mutant (b; n = 9) brains, and immunostained for polyglutamylated tubulin (magenta) and scospondin (green). Inverted, higher magnification images of scospondin immunostaining in forebrain (c, d) and rhombencephalic (e, f) ventricles of control (c, e) and mutant (d, f) brains, represented by boxed areas in a, b. EXPRESSION / LABELING:
PHENOTYPE:
|