- Title
-
Aerobic glycolysis is important for zebrafish larval wound closure and tail regeneration
- Authors
- Scott, C.A., Carney, T.J., Amaya, E.
- Source
- Full text @ Wound Repair Regen.
|
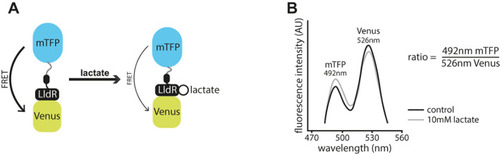
Schematic of the Laconic genetically encoded sensor for lactate. (A) Schematic depiction of the Laconic genetically encoded FRET‐based sensor. A conformational change upon lactate binding to the LldR lactate binding region fused to the Venus chromophore induces a change in energy transfer efficiency from mTFP to Venus (adapted from |
|
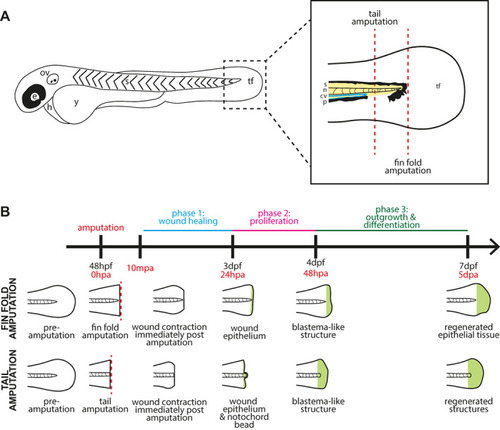
Schematic diagrams of the fin and tail amputation models of zebrafish embryo regeneration. (A) Schematic drawing of a two‐day post fertilisation zebrafish embryo. e: eye, ov: otic vesicle, h: heart, y: yolk, s: somites, tf: tail fin. Dashed box indicates the area shown enlarged in the box to the right, which depicts the tail region with amputation planes for fin fold and tail amputations (dashed red lines). A fin fold amputation site is positioned just distal to the tip of the notochord and excision is limited epithelial tissue and mesenchymal cells, while a tail amputation is oriented using the pigment gap and circulatory loop of the caudal vein, severing notochord, neural tube, and muscle in addition to epithelial tissue. s: somites (yellow), n: notochord (pink), cv: caudal vein (blue), p: pigment (black), tf: tail fin fold. (B) General timeline and schematic illustration of phases of regeneration following amputation. Embryos are imaged at various time points depending on the specific experiment between ten minutes post amputation (10mpa) and full regeneration at five days post amputation (5dpa). |
|
Lactate levels in fin fold regeneration. A) Micrographs of representative |
|
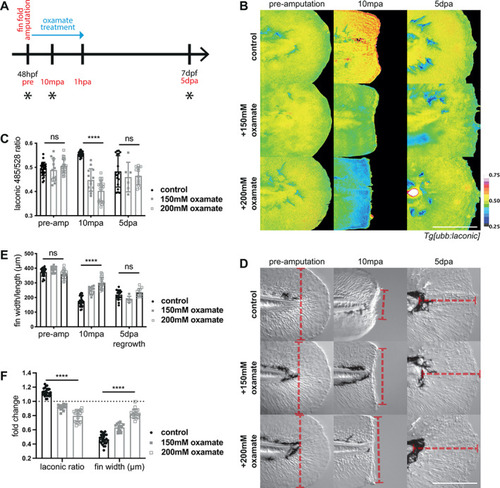
Lactate dehydrogenase inhibition in wound healing. (A) Schematic of the experimental design. Embryos were amputated in the treatment solution and incubated for one hour before washing out the drug, and maintained until regeneration was complete at 120 hpa. Blue arrow indicates period of oxamate treatment and black asterisks indicate time points for imaging. (B) Micrographs of representative |
|
Effect of lactate dehydrogenase inhibition on actomyosin cable contraction in wound healing. (A) Maximal intensity confocal micrograph projections of representative embryos tails at 48 hpf fixed and stained for phospho‐non muscle myosin light chain II (pNM), Actin, DAPI, and merged, at 10 min post amputation. (B) Graphs showing quantification of fluorescence intensity at the wound border of phalloidin Actin staining and immunofluorescent pNM staining. Inset (i) denotes example of region measured. Two‐way ANOVAs used to calculate significance, ** |
|
Mitochondrial inhibition in wound healing. (A) Schematic of the experimental design. Embryos were amputated in the treatment solution and incubated for one‐hour before washing out the drug, and maintained until regeneration was complete at 120 hpa. Green arrow indicates period of sodium azide (NaN3) treatment and black asterisks indicate time points for imaging. (B) Micrographs of representative |
|
Lactate levels in tail regeneration. (A) Micrographs of representative |
|
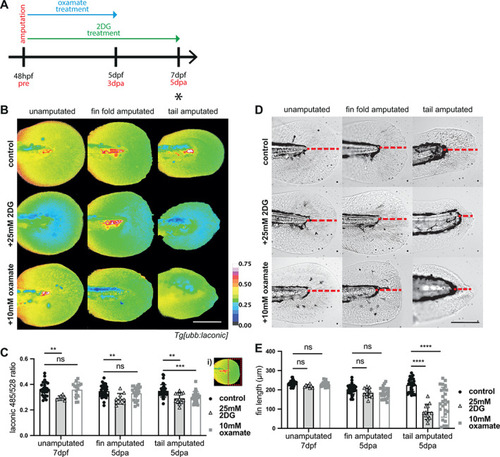
Glycolysis inhibition in regeneration. (A) Schematic of the experimental design. Embryos were amputated and incubated in treatment solution for 72 h or 120 h as indicated by the blue (oxamate) and green (2DG) arrows. Black asterisks indicates time point for imaging, at 5 dpa upon completion of regeneration. (B) Micrographs of representative |