- Title
-
In Situ Fucosylation of the Wnt Co-receptor LRP6 Increases Its Endocytosis and Reduces Wnt/β-Catenin Signaling
- Authors
- Hong, S., Feng, L., Yang, Y., Jiang, H., Hou, X., Guo, P., Marlow, F.L., Stanley, P., Wu, P.
- Source
- Full text @ Cell Chem Biol
|
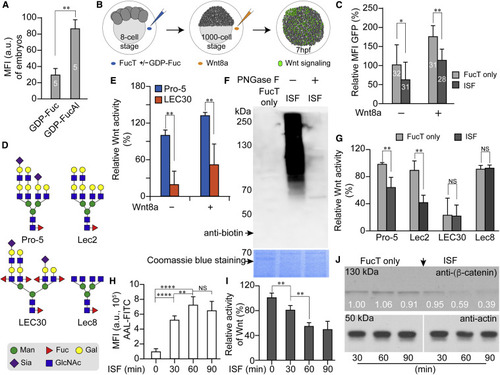
(A) Relative mean fluorescence intensity (MFI) of traditional bio-orthogonal chemical reporter labeling in the FucT/GDP-Fuc- or FucT/GDP-FucAl-treated embryos was quantified by ImageJ. (B) Flow chart of the impacts of ISF on Wnt-signaling activity in a zebrafish Wnt reporter line. At the 8-cell stage, a FucT/GDP-Fuc pre-mixture, or FucT only, were injected into the chorion to perform ISF or act as control, respectively. To further boost the Wnt-signaling readout, we injected recombinant WNT8a into the chorion at the 1,000-cell stage before the embryos were transferred individually into single wells. Expression of the GFP reporter was quantified at 7 hpf. (C) Comparing the GFP expression level of Wnt reporter embryos treated by ISF or FucT only in the presence or absence of WNT8a. The MFI of GFP was quantified for healthy live embryos at 7 hpf by subtracting background in samples with no GFP expression, and the GFP-MFI of embryos treated with FucT only was set as 100%. The numbers on the bar graph represent the numbers of healthy embryos used in this assay. (D) Typical complex N-glycans present on the cell surface glycoproteins of CHO Pro-5 cells, and glycosylation mutants Lec2, LEC30, and Lec8 cells (North et al., 2010). Pro-5 and Lec2 cells carry LacNAc with no Fuc attached and are excellent substrates for ISF. Lec8 has no LacNAc, and LEC30 has many fucosylated LacNAcs. Sugar symbols are from the Symbol Nomenclature for Glycans (Neelamegham et al., 2019). (E) Comparison of Wnt activity in Pro-5 and LEC30 cells in the presence or absence of WNT8a. (F) Western blot assay for detection of biotinylated glycoproteins in Pro-5 cells that generated via GDP-Fuc-biotin-ISF before or after PNGase F-treatment. Equal loading determined by Coomassie blue staining (lower panel). (G) Comparison of Wnt activity in CHO parent and three mutant lines treated with ISF or FucT only. (H) Quantitative flow-cytometry analysis of time-dependent incorporation of α(1–3)-Fuc onto Pro-5 cell-surface glycans via ISF treatment. (I) Time-dependent decrease of Wnt activity in Pro-5 cells treated by ISF. (J) Time-dependent decrease of the β-catenin level in ISF-treated Pro-5 cells. Western blot of anti-β-catenin (upper panel) and actin (lower panel). The numbers indicate the relative quantification of anti-β-catenin level to actin by ImageJ. The 30-min, non-treated sample was set as 100%. Number (in white) indicates the relative β-catenin level normalized to the corresponding level of actin. In (A) and (B), results are from the same set of samples and the signal intensity of sample at 0-min treatment is set as 100%. Error bars represent the SD of three biological replicates. Unpaired two-tailed Student's t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; NS, not significant. |
|
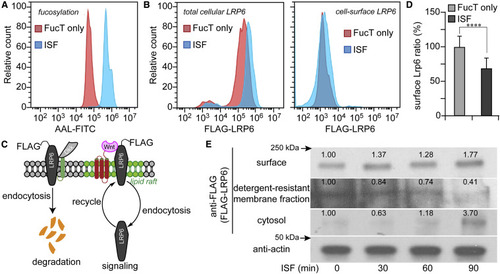
(A and B) Flow-cytometry histograms of FucT-treated (red) and ISF-treated (blue) Pro-5 CHO cells after 90 min of incubation. LRP6 in (B) was transiently-expressed FLAG-LRP6. (A) Detection of cell surface N-glycan LacNAc fucosylation by the lectin AAL-FITC. (B) Immunofluorescent staining of total cellular LRP6 and cell-surface LRP6. (C) Scheme of LRP6 location and maintenance regulated by endocytosis. (D) Ratio of LRP6 (surface to total) in Pro-5 cells treated by ISF or FucT only. Mock control was set as 100%. Error bars show standard error. N = 53,664 cells. Unpaired two-tailed Student's t test: ∗∗∗∗p < 0.001. (E) Western blot assay of membrane LRP6, lipid raft LRP6, cytoplasmic LRP6, and actin after different times of ISF treatment. Number indicates the relative LRP6 level normalized to the corresponding level of actin. |
|
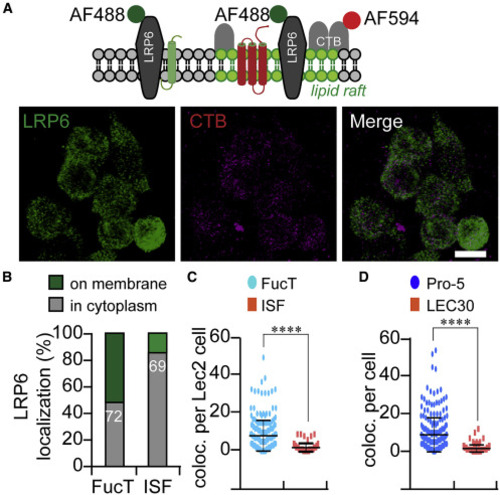
(A) Schematic representation (top panel) of the membrane topological location of the lipid-raft markers CTB and LRP6. The co-localization of LRP6 and CTB (gray dots) in lipid rafts was calculated from confocal images using ImageJ, based on immunofluorescence of antibody to LRP6 (green) and labeled CTB (red). Scale bar, 20 μm. (B) Comparing the co-localization of LRP6 and CTB showed that LRP6 moved from membrane to cytoplasm in ISF-treated Pro-5 cells. The numbers show the cells counted in this assay. (C and D) Plots of the co-localization of LRP6 and CTB (per cell) in ISF-treated and FucT-treated Lec2 cells (C) or in untreated LEC30 cells (D). Unpaired two-tailed Student's t test: ∗∗∗∗p < 0.001. |
|
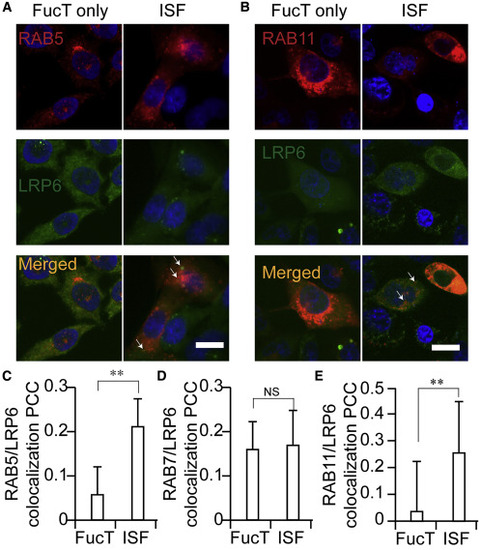
(A and B) Confocal visualization of LRP6 (FLAG-LRP6, green) in FucT only-treated and ISF-treated Pro-5 cells. Early endosome marker RAB5-mCherry (A, red) and recycling endosome marker RAB11-mCherry (B, red) were transiently expressed in Pro-5 cells. The co-localization of LRP6 with RAB5 or RAB11 is shown in yellow. The white arrows indicate the presentative co-localization of LRP6 with RAB5 or RAB11, respectively. Scale bars, 20 μm. (C–E) Co-localization of RAB5 (C), RAB7 (D), or RAB11 (E) with LRP6 in FucT-only-treated and ISF-treated Pro-5 cells, is shown as Pearson's correlation coefficient. Two-sided Student's t test: ∗∗p < 0.01; NS, not significant. |
|
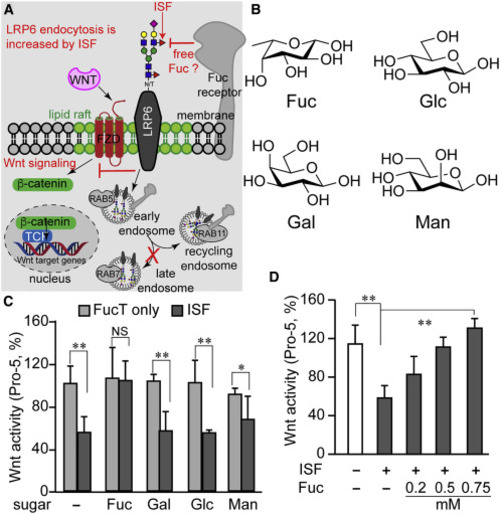
(A) Diagram summarizing our new findings and predicting the presence of a Fuc-binding receptor. Increasing cell-surface N-glycan LacNAc α(1–3)-fucosylation via ISF treatment exacerbates the endocytosis of the lipid-raft-localized Wnt receptor LRP6 which, in turn, leads to reduced Wnt/β-catenin signaling. The majority of endocytosed cell-surface LRP6 enters the recycling pathway rather than the degradation pathway following endocytosis induced by ISF treatment. (B) The chemical structure of four monosaccharides in a vertebrate N-glycan: L-fucose (Fuc), D-glucose (Glc), D-galactose (Gal), and D-mannose (Man). (C and D) Wnt-signaling activity in Pro-5 cells treated by ISF or FucT only, in the presence (C) or absence (D) of a free monosaccharide. Error bars represent SE of three biological replicates. Unpaired two-tailed Student's t test: ∗p < 0.05, ∗∗p < 0.01; NS, not significant. |
|
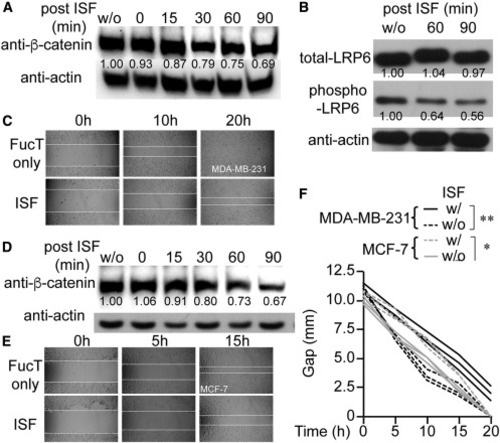
(A) Time-dependent decrease of the β-catenin level in ISF-treated MDA-MB-231. Western blot of anti-β-catenin (upper panel) and anti-actin (lower panel). (B) Immunoprecipitation of LRP6 from indicated MDA-MB-231 cells and detection of LRP6 and phosphorylated LRP6 by immunoblot. ISF-dependent decrease of LRP-6 phosphorylation. (C) Wound-healing assay results from MDA-MB-231 cells treated with ISF or not. (D) Time-dependent decrease of the β-catenin level in ISF-treated MCF-7. (E) Wound healing of MCF-7 cells treated with ISF or not. (F) Time-dependent gap closure of MDA-MB-231 and MCF-7 cells treated with ISF or not in wound-healing assays. One-way ANOVA: ∗p < 0.05, ∗∗p < 0.01. In (A), (B), and (D), the number indicates the relative protein level normalized to the corresponding level of actin. |