- Title
-
Dhx15 regulates zebrafish definitive hematopoiesis through the unfolded protein response pathway
- Authors
- Cai, Y., Wang, J., Jin, D., Liu, Q., Xianglei, C., Lili, P., Yang, L., Wang, X., Qian, F., Wang, J., Zhong, T.P., Wang, S.
- Source
- Full text @ Cancer Sci.
|
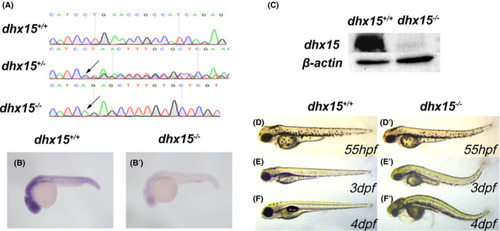
Generation of dhx15−/− zebrafish. A, DNA sequencing results of dhx15+/+ , dhx15+/− , and dhx15−/− embryos. Arrows indicate a 13 bp deletion (CTGAACCGCCATC) at dhx15 exon 2. B, B’, WISH analysis of dhx15 in dhx15+/+ and dhx15−/− embryos at 24 hpf. C, Western blot analysis of dhx15 in dhx15+/+ and dhx15−/− embryos at 3 dpf. D‐F’, Bright‐field microscopy images of dhx15+/+ and dhx15−/− zebrafish. dpf, days post fertilization; hpf, hours post fertilization; WISH, whole‐mount in situ hybridization PHENOTYPE:
|
|
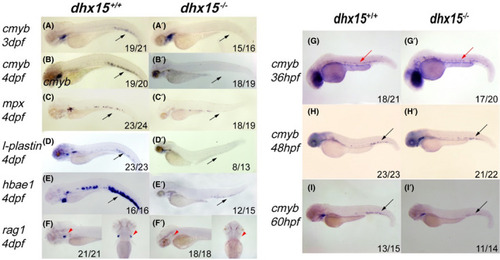
Dhx15−/− zebrafish displayed defective definitive hematopoiesis in CHT. A‐B’, WISH analysis of cmyb expression in dhx15+/+ and dhx15−/− at 3 dpf (A, A’) and 4 dpf (B, B’). C‐F’, WISH analysis of mpx (C, C’), l‐plastin (D, D’), hbae1 (E, E’), rag1 (F, F’) expression in dhx15+/+ and dhx15−/− zebrafish. G‐I’, WISH analysis of cmyb expression in dhx15+/+ and dhx15−/− zebrafish at 36 hpf (G, G’), 48 hpf (H, H’), 60 hpf (I, I’). Black arrows indicate CHT; red arrows indicate ventral wall of dorsal aorta; red arrowheads indicate thymus. CHT, caudal hematopoietic tissue; dpf: days post fertilization; hpf, hours post fertilization; WISH, whole‐mount in situ hybridization |
|
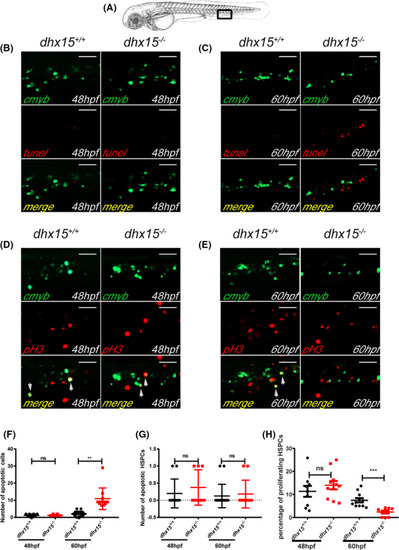
Abated proliferation but not excessive apoptosis of HSPCs is observed in dhx15−/− zebrafish. A, Schematic indicates the area of the imaging in the tail (CHT), outlined by the black box. B, C, Fluorescence images of the cmyb and apoptotic cells in CHT of dhx15+/+; Tg(cmyb:gfp) and dhx15−/−; Tg(cmyb:gfp) zebrafish embryos at 48 hours post fertilization (hpf) (B) and 60 hpf (C). D, E, Fluorescence images of pH3 and cmyb positive cells in CHT of dhx15+/+; Tg(cmyb:gfp) and dhx15−/−; Tg(cmyb:gfp) zebrafish embryos at 48 hpf (D) and 60 hpf (E). F, Number of apoptotic cells in CHT at 48 hpf (wildtype, 1.2 ± 0.2494, n = 10; dhx15−/− embryos, 1.25 ± 0.1637, n = 8; P > .05, Student’s t test) and 60 hpf (wildtype, 2.063 ± 0.3923, n = 16; dhx15−/− embryos, 10.91 ± 1.944, n = 11; P < .005, Student’s t test). G, Number of apoptotic HSPCs in CHT at 48 hpf (wildtype, 0.2 ± 0.1333, n = 10; dhx15−/− embryos, 0.375 ± 0.183, n = 8; P > .05, Student’s t test) and 60 hpf (wildtype, 0.125 ± 0.08539, n = 16; dhx15−/− embryos, 0.1818 ± 0.122, n = 11; P > .05, Student’s t test). H, Percentages of proliferating HSPCs in CHT at 48 hpf (wildtype, 11.34 ± 2.379, n = 9; dhx15−/− embryos, 13.98 ± 1.829, n = 11; P > .05, Student’s t test) and 60 hpf (wildtype, 7.464 ± 0.9413, n = 11; dhx15−/− embryos, 2.367 ± 0.6178, n = 9; P < .0005, Student’s t test). Data are represented as mean ± SEM. *P < .05; **P < .005; ***P < .0005. Scale bar = 40 μm. CHT, caudal hematopoietic tissue; hpf, hours post fertilization; HSPCs, hematopoietic stem/progenitor cells; ns, not significant; pH3, pospho‐histone H3 |
|
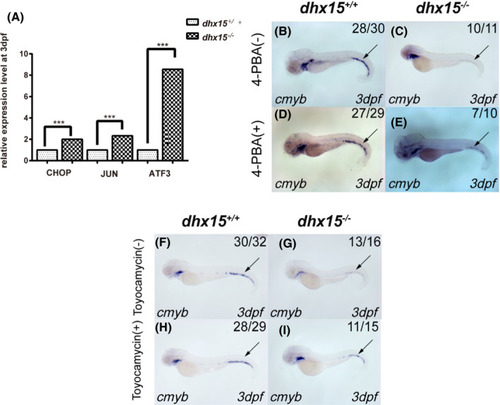
UPR pathway mediates HSPC failure in dhx15−/− zebrafish. A, qRT‐PCR analysis of CHOP, JUN, and ATF3 in dhx15+/+ and dhx15−/− zebrafish at 3 dpf. Each qRT‐PCR experiment was carried out in triplicates, and repeated at least three times. B‐E, WISH analysis of cmyb in dhx15+/+ and dhx15−/− zebrafish treated with 4‐PBA. P < .005, χ2 test. F‐I, WISH analysis of cmyb in dhx15+/+ and dhx15−/− zebrafish treated with toyocamycin. P < .005, χ2 test. Black arrow indicates caudal hematopoietic tissue. ***P < .0005. ATF3, activating transcription factor 3; dpf, days post fertilization; HSPC, hematopoietic stem/progenitor cell; qRT‐PCR, quantitative real‐time PCR; UPR, unfolded protein response pathway; WISH, whole‐mount in situ hybridization; 4‐PBA, 4‐phenylbutyric acid EXPRESSION / LABELING:
PHENOTYPE:
|
|
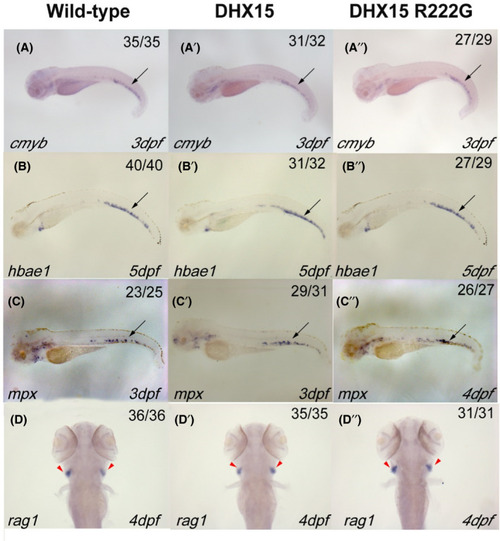
Definitive hematopoiesis in zebrafish is normal when DHX15 or DHX15 R222G is overexpressed. WISH analysis of definitive hematopoietic markers: cmyb (A‐A’’), hbae1 (B‐B’’), mpx (C‐C’’), and rag1 (D‐D’’). Embryos from wildtype, Tg(hsp: DHX15), and Tg(hsp: DHX15 R222G) zebrafish were heat‐shocked at 38℃ for 2 h every day since 25 hours post fertilization (hpf). DHX15, Tg(hsp: DHX15); DHX15 R222G, Tg(hsp: DHX15 R222G). Black arrows indicate caudal hematopoietic tissue; red arrowheads indicate thymus. hpf, hours post fertilization; WISH, whole‐mount in situ hybridization EXPRESSION / LABELING:
PHENOTYPE:
|
|
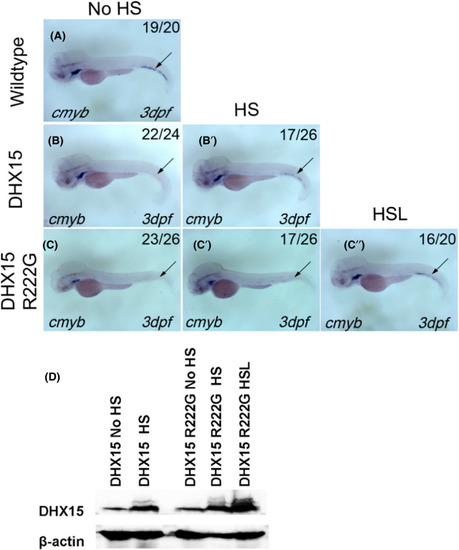
DHX15 R222G is not as effective as DHX15 in restoring HSPC failure in dhx15−/− zebrafish. A, cmyb expression of wildtype zebrafish at 3 dpf. B, B’, cmyb expression of Dhx15−/−; Tg(hsp: DHX15) zebrafish at 3 dpf with (B’) or without (B) heat‐shock treatment. HSPC failure was rescued after DHX15 overexpression in dhx15−/− zebrafish (P < .0001, χ2 test). C‐C’’, cmyb expression of Dhx15−/−; Tg(hsp: DHX15 R222G) zebrafish at 3 dpf with (C’, C’’) or without (C) HS treatment. HSPC failure was rescued after DHX15 R222G overexpression in dhx15−/− zebrafish (P < .05, χ2 test). DHX15 R222G is not as effective as DHX15 in restoring HSPC failure in dhx15−/− zebrafish. (No HS vs HS, P < .05, χ2 test; No HS vs HSL, P < .0001, χ2 test; HS vs HSL, P < .005, χ2 test). D, Western blot analysis of DHX15 protein expression in DHX15/DHX15 R222G transgenic zebrafish with or without heat activation. DHX15, Dhx15−/−; Tg(hsp: DHX15); DHX15 R222G, Dhx15−/− ; Tg(hsp: DHX15 R222G); dpf, days post fertilization; hpf, hours post fertilization; HS, heat‐shocked at 38℃ for 1 h and 45 min once a day since 25 hpf; HSL, heat‐shocked at 38℃ for 2 h once a day since 25 hpf; HSPC, hematopoietic stem/progenitor cell; No HS, without heat‐shock treatment. Black arrow indicates caudal hematopoietic tissue EXPRESSION / LABELING:
PHENOTYPE:
|