- Title
-
Stimulation of hepatocarcinogenesis by activated cholangiocytes via Il17a/f1 pathway in kras transgenic zebrafish model
- Authors
- Helal, M., Yan, C., Gong, Z.
- Source
- Full text @ Sci. Rep.
|
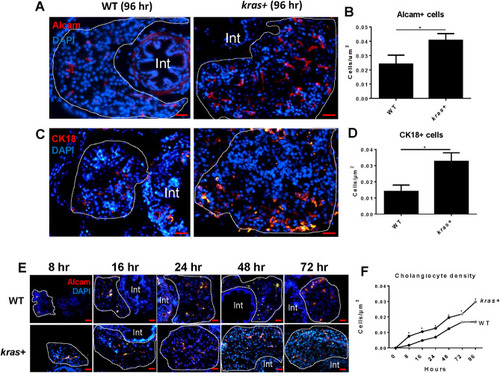
Increase of cholangiocytes upon induction of oncogenic EXPRESSION / LABELING:
PHENOTYPE:
|
|
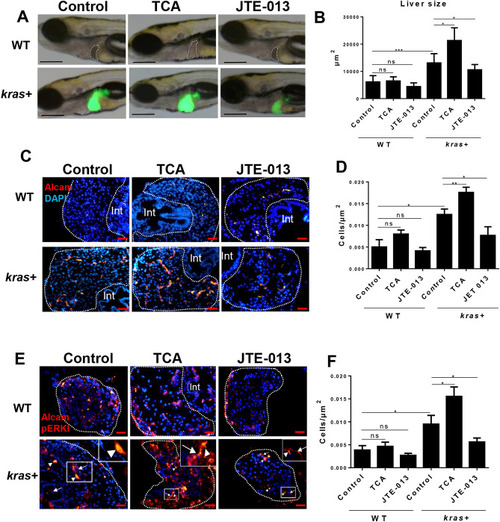
Effect of S1pr2 activation and inhibition on liver size, cholangiocyte density, and downstream marker pERK in |
|
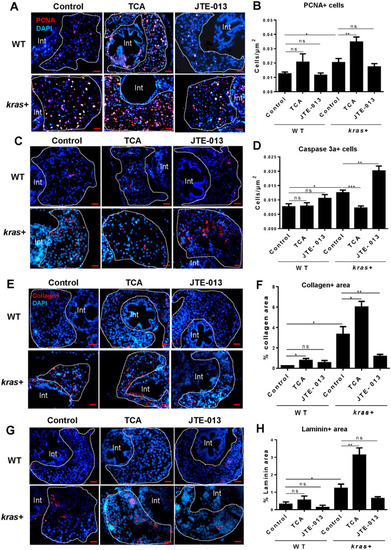
Effect of cholangiocyte activation and inhibition on hepatocyte proliferation, apoptosis and fibrosis. 3-dpf |
|
Effects of differential feeding on liver tumorigenesis. 7-dpf EXPRESSION / LABELING:
PHENOTYPE:
|
|
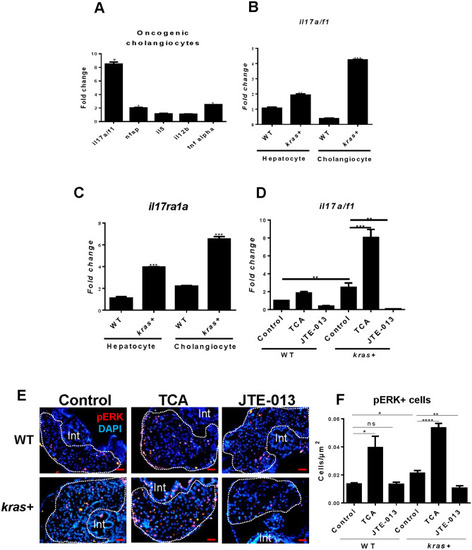
Expression of selected cytokine mRNAs in hepatocytes and cholangiocytes of the liver in adult zebrafish. ( |
|
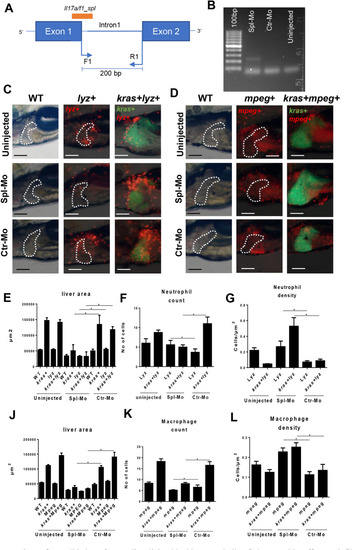
Validation of PHENOTYPE:
|
|
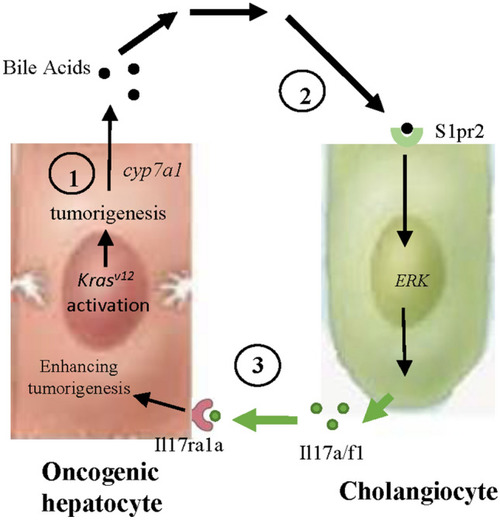
Proposed model for interaction between oncogenic hepatocytes and cholangiocytes in the |