- Title
-
Thyroid hormone receptor beta mutations alter photoreceptor development and function in Danio rerio (zebrafish)
- Authors
- Deveau, C., Jiao, X., Suzuki, S.C., Krishnakumar, A., Yoshimatsu, T., Hejtmancik, J.F., Nelson, R.F.
- Source
- Full text @ PLoS Genet.
|
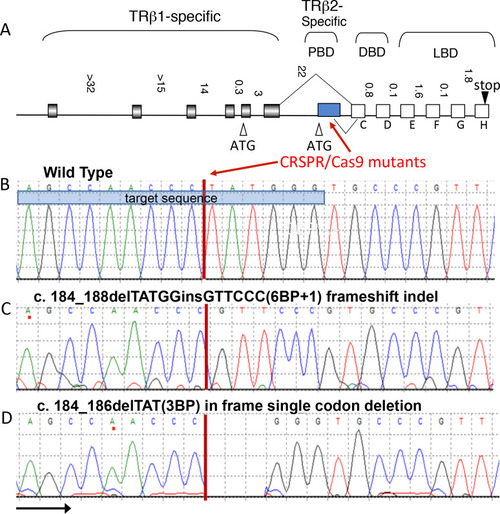
(A) Both mutations are in the first exon of the |
|
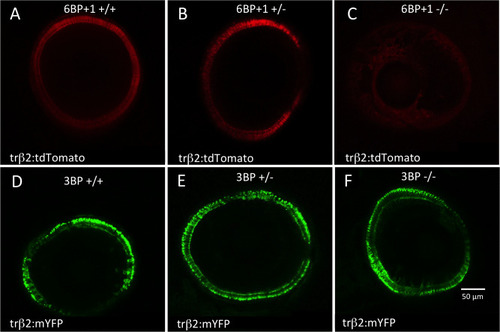
(A) A confocal cross section of a wild type ( |
|
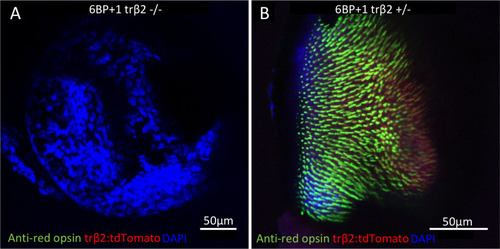
(A) Mutant retina did not have anti-red-opsin or |
|
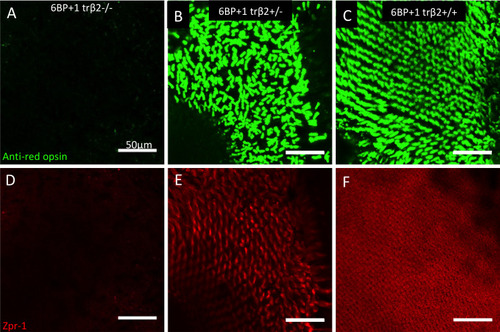
(A, D) Mutant adult retinas (N = 4) did not have red opsin or arr3a (red-green double-cone) antibody fluorescence. (B, E) Heterozygote adult retinas (N = 4) showed bright antibody staining for red opsin and arrestin3a as seen in the wild type (N = 4) (C, F). Scale is the same for each image. Opsin and arr3a images are from the same microscope field, but different planes of focus. Staining was repeated twice for each genotype. |
|
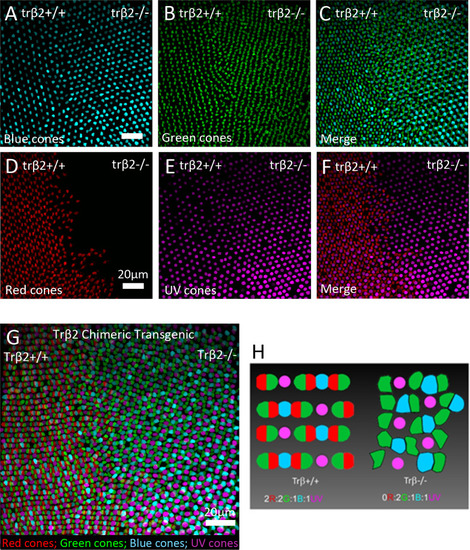
Left side of each image is either wild type or |
|
(A) The PHENOTYPE:
|
|
(A) The PHENOTYPE:
|
|
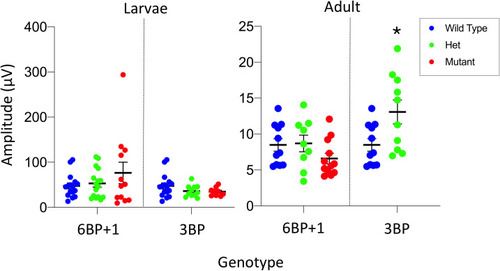
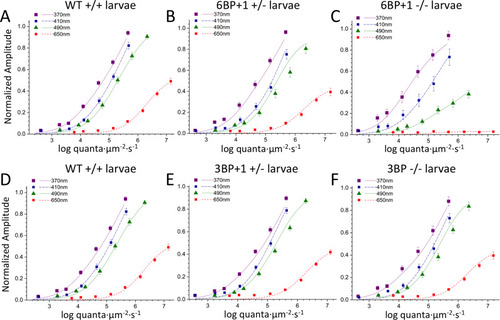
The maximum cone PIII amplitudes for datasets from each genotype are compared. There is no significant difference between the |
|
(A, B) The wild type (N = 17 datasets, 1190 points total) and PHENOTYPE:
|
|
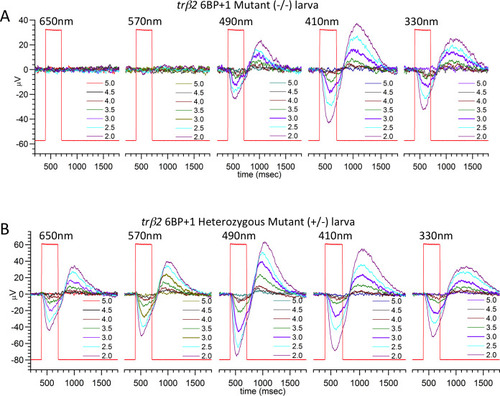
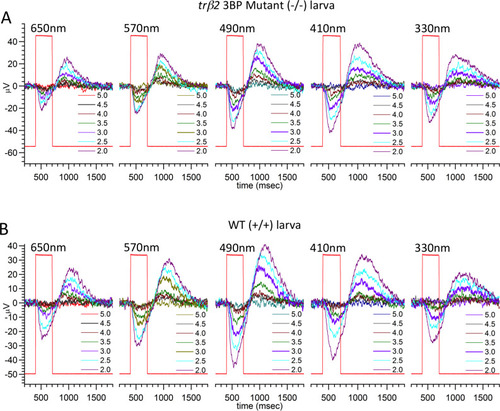
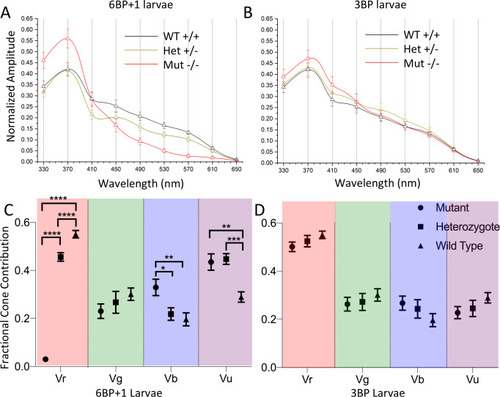
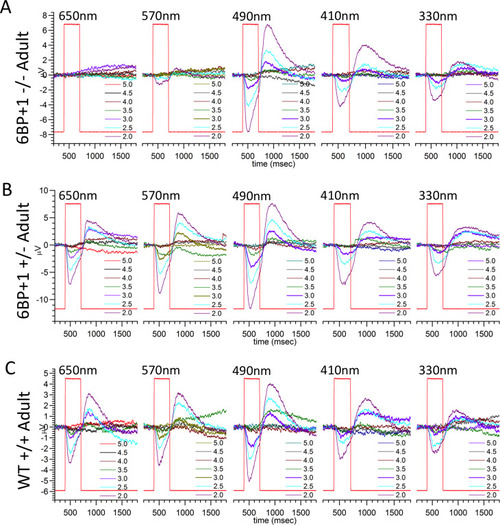
(A) Scanning from 330 nm to 650 nm with constant quantal irradiance (4.6 log(quanta∙μm−2∙s−1) the PHENOTYPE:
|
|
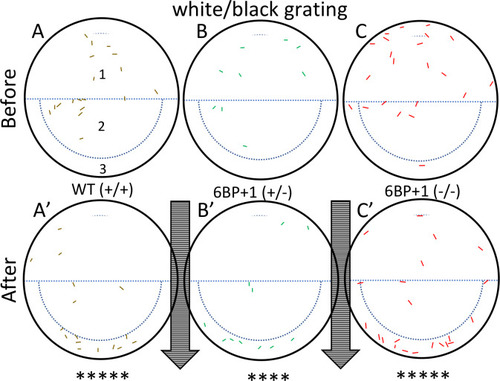
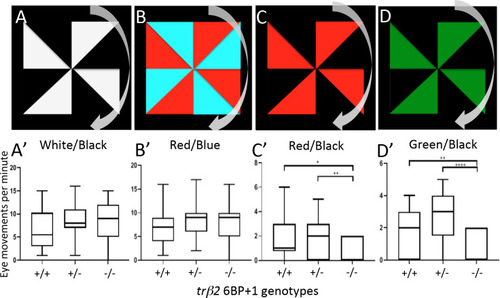
Controls use a drifting black and white grating pattern with wild types (+/+) (N = 18 larvae), |
|
The wild type (N = 18 larvae) (A, A’) and heterozygote (N = 12 larvae) (B, B’) larva moved with the stimulus as seen in the white/black drifting gratings ( PHENOTYPE:
|
|
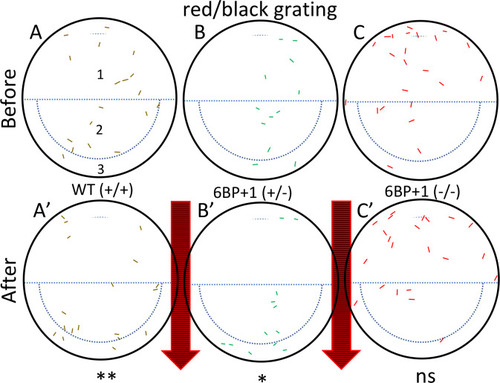
(A, B, C, D) Each larva at 9 dpf was exposed to these four stimuli in left to right order. The pinwheel patterns moved clockwise. (A’, B’) The wild type, PHENOTYPE:
|
|
(A) PHENOTYPE:
|
|
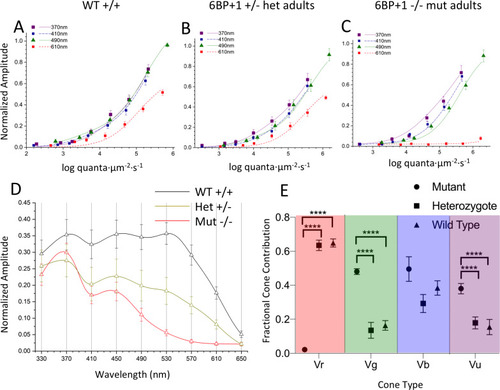
(A, B, C) The irradiance plots for each genotype showed the loss of response to 610 nm in the mutant regardless of brightness. (D) The spectral plot showed a significant loss of amplitude in the heterozygote (N = 9) and mutant (N = 12) at long wavelengths compared to the WT (N = 11). (E) The mutant shows loss of Vr, increase in Vg, and increase in Vu. PHENOTYPE:
|
|
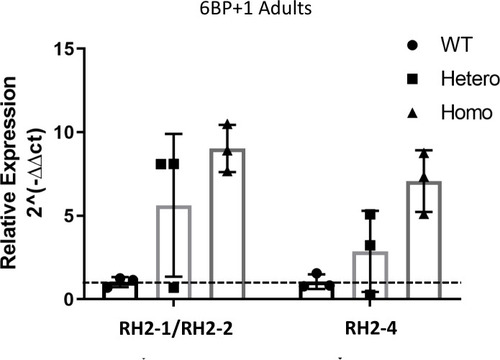
The expression levels of combined |

Unillustrated author statements |