- Title
-
Chemokine receptor trafficking coordinates neutrophil clustering and dispersal at wounds in zebrafish
- Authors
- Coombs, C., Georgantzoglou, A., Walker, H.A., Patt, J., Merten, N., Poplimont, H., Busch-Nentwich, E.M., Williams, S., Kotsi, C., Kostenis, E., Sarris, M.
- Source
- Full text @ Nat. Commun.
|
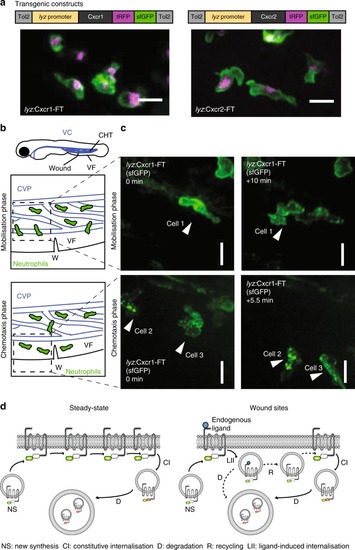
Live imaging of chemokine receptor trafficking in neutrophils. EXPRESSION / LABELING:
|
|
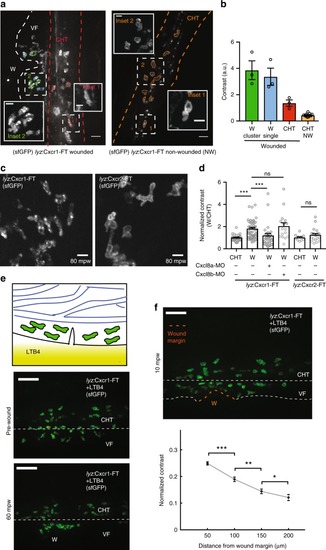
Distinct trafficking of Cxcr1 and Cxcr2 during neutrophil migration to wounds. PHENOTYPE:
|
|
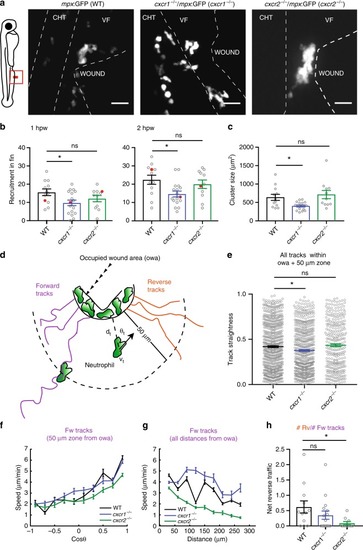
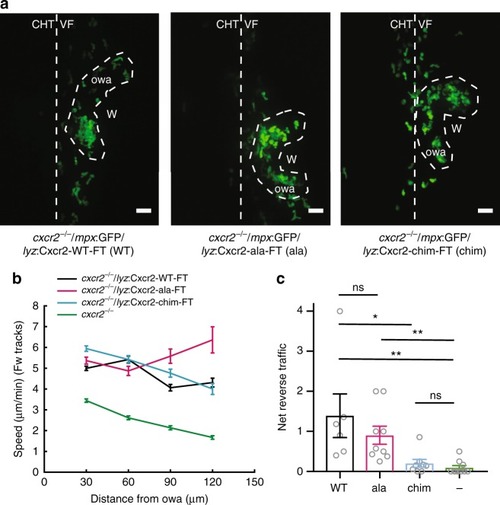
Differential contributions of Cxcr1 and Cxcr2 in neutrophil clustering and dispersal. PHENOTYPE:
|
|
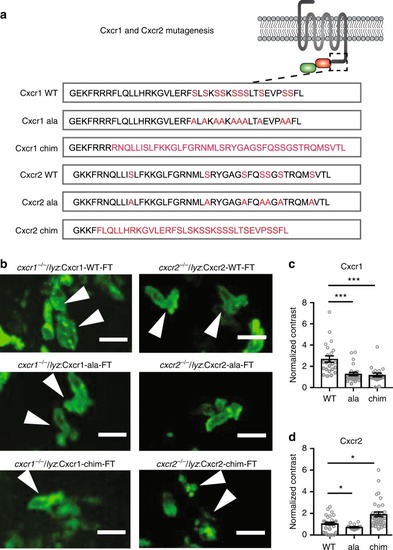
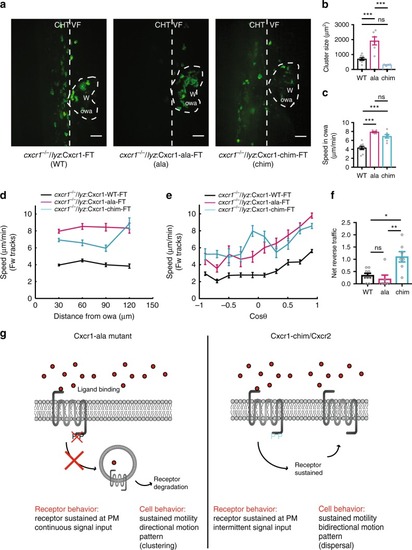
Receptor mutagenesis alters Cxcr1 and Cxcr2 trafficking. |
|
Plasma membrane sustenance of Cxcr2 is required for neutrophil dispersal. |
|
Receptor internalization limits neutrophil motion at wounds. |
|
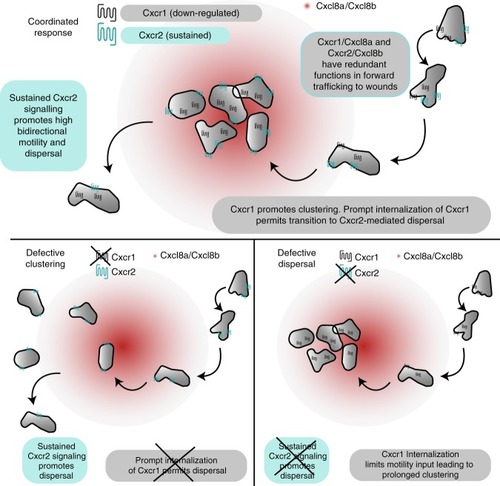
Model for coordination of neutrophil clustering and dispersal through chemokine receptor trafficking. (Top) Cxcr1/Cxcl8a and Cxcr2/Cxcl8b can partly compensate for each other during initial chemotaxis to the wounded tissue. However, Cxcr1 specifically promotes clustering and this contribution is limited by desensitization and downregulation. Conversely, Cxcr2 is recycled after internalization and promotes persistent bidirectional motility in the wounded tissue through sustained, intermittent signaling. This facilitates dispersal from the site. Bottom left: in the absence of Cxcr1, neutrophils are recruited, through Cxcr2/Cxcl8b and other endogenous signals, but show a loss in clustering. Bottom right: in the absence of Cxcr2, neutrophils are recruited, through Cxcr1/Cxcl8a and other endogenous signals. Once at the target, Cxcr1 is maximally downregulated and neutrophils lack signal input for motility, leading to a defect in dispersal |