- Title
-
The miR-216a-Dot1l Regulatory Axis Is Necessary and Sufficient for Müller Glia Reprogramming during Retina Regeneration
- Authors
- Kara, N., Kent, M.R., Didiano, D., Rajaram, K., Zhao, A., Summerbell, E.R., Patton, J.G.
- Source
- Full text @ Cell Rep.
|
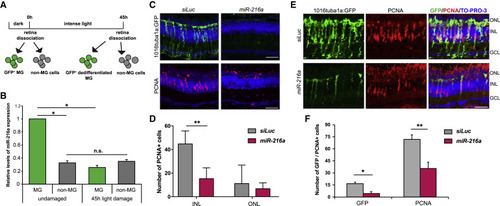
Suppression of miR-216a Is Required for MG Dedifferentiation and Proliferation during Retina Regeneration (A) Schematic for post-mitotic and dedifferentiated MG sorting. Adult zebrafish were dark adapted for 2 weeks and then exposed to constant intense light lesioning for 45 h. For post-mitotic MG isolation, GFP+cells were sorted from dark adapted Tg(gfap:gfp)retinas. For dedifferentiated MG isolation, GFP+ cells were isolated from 45 h light lesioned Tg(1016tuba1a:GFP)retinas. (B) Fold changes in miR-216a levels in FACS-purified MG were determined by qPCR. miR-216a is enriched in post-mitotic MG (GFP+) from undamaged retinas in Tg(gfap:gfp) fish. After 45 h of light damage, miR-216ais downregulated ∼5-fold in dedifferentiated MG (GFP+) in Tg(1016tuba1a:gfp) fish. miR-216a expression did not change in non-MG cells (GFP−) during regeneration. Data are from 5 independent experiments with 3 technical replicates of qPCR. MG were purified from 18 and 20 light-damaged fish in each experiment. Error bars represent the SEMs. ∗p < 0.05 and ∗∗p < 0.01 using 2-way ANOVA with Fisher’s least significant difference (LSD) post hoc test. (C) Control miRNA (siRNA against luciferase [siLuc]) or miR-216a was injected and electroporated into the left eyes of Tg(1016tuba1a:gfp) zebrafish before intense light exposure (0 h). After 45 h, retinas were collected, sectioned, and immunostained using antibodies against GFP and PCNA. Nuclei were counterstained with TOPRO (blue). miR-216again of function abolished tuba1a:GFP transgene expression and significantly reduced the number of INL PCNA+ proliferating cells. (D) Quantification of PCNA+ cells in the INL and ONL. Error bars represent the SEMs (n = 5–6 fish); ∗∗p < 0.01 using Student’s t test. (E) Overexpression of miR-216a reduced the number of GFP+/PCNA+proliferating progenitor cells after 60 h of intense light damage using Tg(1016tuba1a:gfp) zebrafish. (F) Quantification of total GFP+ and PCNA+ cells. Error bars represent the SEMs (n = 10 fish); ∗p < 0.03 and ∗∗p < 0.003 using Student’s t test. GCL, ganglion cell layer; INL, inner nuclear layer; ns, not significant; ONL, outer nuclear layer. Scale bars, 50 μm. |
|
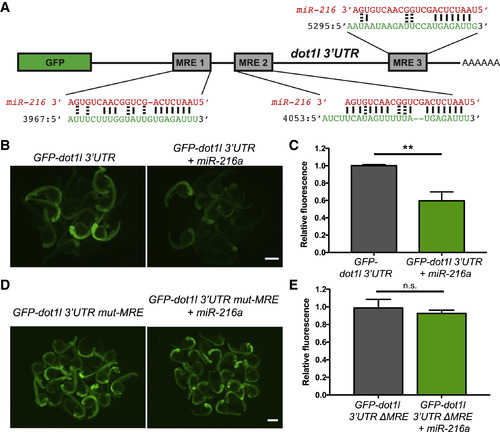
Dot1l Is a Direct Target of miR-216a (A) Schematic of the reporter mRNA consisting of the coding sequence of GFP fused to the dot1l 3′ UTR. Three predicted miRNA recognition elements (MREs) are indicated. Predicted base pairing between MREs (shown in green) and the miR-216a sequence (shown in red). (B) Embryos injected at the 1-cell stage with 100 pg of GFP-dot1l 3′ UTR, with or without 100 pg miR-216a, were examined for GFP expression at 1 day post fertilization (dpf). GFP expression was apparent in embryos injected with GFP-dot1l 3′ UTR, but it was reduced in embryos co-injected with miR-216a. (C) Quantification of relative fluorescence in 1 dpf embryos injected with GFP reporter only or co-injected with miR-216a and the GFP reporter. Data represent means ± SEMs from 3 independent experiments. ∗∗p < 0.01 (Student’s t test). (D) One-dpf-old embryos injected at the 1-cell stage with 100 pg of GFP-dot1l 3′ UTR carrying mutations in all miR-216a MREs. Embryos were injected with the mutant reporter, either alone or co-injected with miR-216a. (E) Quantification of relative fluorescence in 1 dpf embryos injected with mutant GFP reporter alone or with co-injection of miR-216a. Data represent means ± SEMs from 3 independent experiments. ∗∗p < 0.01 (Student’s t test). Scale bars, 500 μm. |
|
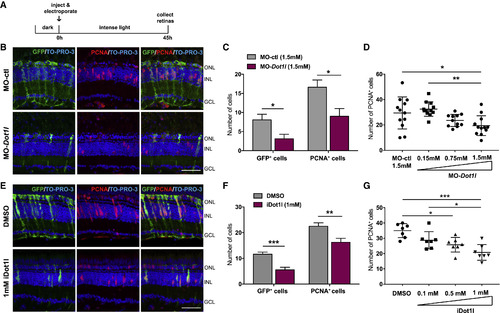
Dot1l Is Required for MG Dedifferentiation and Proliferation during Retina Regeneration (A) Control MO or dot1lMOs were injected and electroporated into the left eyes of Tg(1016tuba1a:gfp) zebrafish before intense light exposure (0 h). After 45 h, retinas were collected, sectioned, and immunostained using antibodies against GFP and PCNA. Nuclei were counterstained with TOPRO (blue). (B) Dot1l loss-of-function reduced tuba1a:GFP transgene expression and the number of INL PCNA+proliferating cells. (C) Quantification of GFP+dedifferentiated MG and PCNA+ proliferating progenitors in MO-ctl and MO-dot1l electroporated retinas. Data represent means ± SEMs, n = 5–6 fish; ∗∗p < 0.01 by 2-tailed Mann-Whitney U test. (D) Dose response to MO-dot1l. Increasing amounts of MO-dot1l MOs were injected and analyzed as in (B) and (C). Each data point represents an individual fish; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 by 1-way ANOVA. (E) Control vehicle (DMSO) or iDot1l (Dot1l inhibitor EPZ004777) were injected intravitreally into the left eyes of Tg(1016tuba1a:gfp) zebrafish before intense light exposure (0 h). After 45 h, retinas were collected, sectioned, and immunostained using antibodies against GFP and PCNA. Nuclei were counterstained with TOPRO (blue). (F) Quantification of total GFP+ and PCNA+ cells. Data represent means ± SEMs, n = 10 fish; ∗p = 0.0111; ∗∗∗∗p < 0.0001 by 2-tailed Mann-Whitney Utest. (G) Dose response to iDot1l. Increasing amounts of the Dot1l inhibitor were injected and analyzed as in (E) and (F). Each data point represents an individual fish; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 by 1-way ANOVA. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer;. Scale bars, 50 μm. See also Figures S2 and S3. |
|
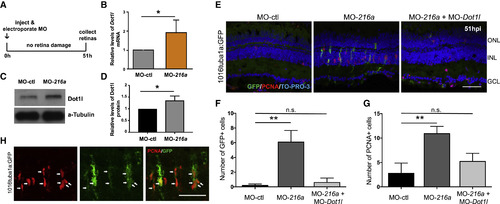
miR-216aSuppression Stimulates MG Dedifferentiation and Proliferation in the Uninjured Retina through Regulating Dot1l (A) Undamaged Tg(1016tuba1a:gfp)zebrafish were injected and electroporated with control MOs (n = 5), miR-216aMOs (n = 6), or both (n = 5). Either eyes for immunostaining or retinas for expression analysis were collected at 51 h post-injection (hpi). (B) Fold changes in dot1llevels in MO-ctl or MO-216a electroporated retinas were quantified by qPCR. At 51 hpi, dot1l was significantly upregulated in miR-216a overexpressing retinas ∼2-fold. Data represent means ± SEMs; ∗p < 0.05 using Student’s t test, p = 0.0365. (C) Representative western blot for Dot1l and α-tubulin in control and miR-216aMO-injected retinas at 51 hpi. (D) Quantification for the relative levels of Dot1l protein in miR-216a MO-injected retinas compared to controls. Data represent means ± SEMs from 3 independent experiments, ∗p < 0.05 using Student’s t test. (E) miR-216a MOs significantly increased the number of PCNA+ and GFP+ cells in the INL, while there was no significant difference between miR-216a MO + dot1l MO co-injected eyes and control eyes. (F and G) Quantification of GFP+ dedifferentiated MG (F) and PCNA+ proliferating progenitors (G) in MO-ctl, MO-216a, and MO-216a + MO-dot1l electroporated retinas. Data represent means ± SEMs (n = 6 fish). ∗∗p < 0.01, PCNA p = 0.0098, and GFP p = 0.0039 by 1-way ANOVA with Dunnett’s multiple comparisons test. (H) Representative retinal sections showing colocalization of tuba1a-GFP+ and PCNA+ cells in miR-216 MO injected undamaged retinas at 51 hpi. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 50 μm. |
|
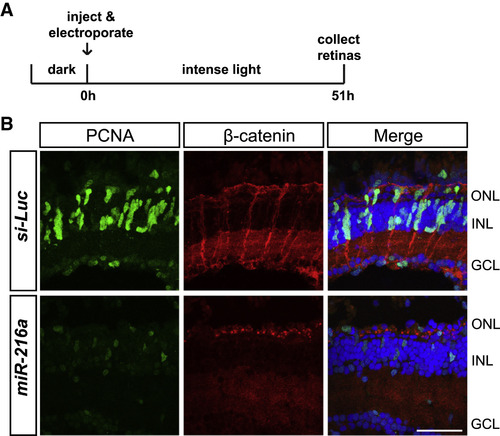
miR-216a Gain of Function Impairs β-Catenin Accumulation in MG after Intense Light Damage (A) Control miRNA or miR-216a was injected and electroporated into the left eyes of wild-type zebrafish before intense light exposure (0 h). After 45 h, retinas were collected, sectioned, and immunostained using antibodies against β-catenin (red) and PCNA (green). Nuclei were counterstained with TOPRO (blue). (B) β-Catenin colocalized with PCNA+ proliferating MG after 45 h of intense light damage. β-Catenin accumulation was not detected in miR-216a-overexpressing retinas. Scale bar, 50 μm. |
|
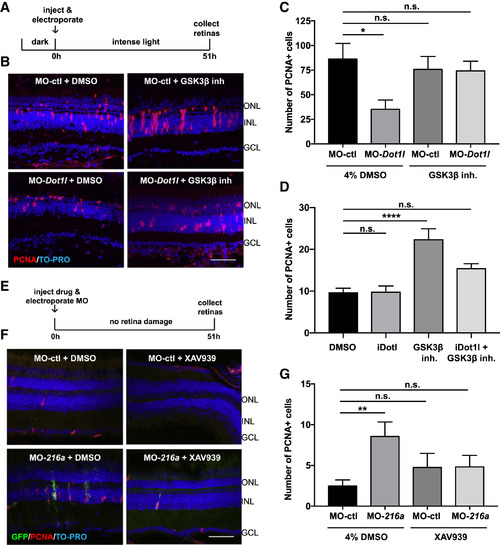
miR-216a and Dot1l Regulate Retinal Regeneration through the Wnt/β-Catenin Pathway (A) Control MOs or Dot1l MOs were injected and electroporated into the left eyes of wild-type zebrafish, followed by either 4% DMSO or GSK-3β-inhibitor (1 mM) before intense light exposure (0 h). Eyes were collected after 51 h of intense light lesioning and immunostained using antibodies against PCNA. Nuclei were counterstained with TOPRO (blue). (B) dot1l MOs significantly decreased the number of PCNA+ cells in the INL, while there was no significant difference between dotl1l MO + GSK-3β-inhibitor co-injected eyes and control eyes. (C) Quantification of PCNA+ proliferating progenitors in MO-ctl + DMSO, MO-dot1l + DMSO, MO-ctl + GSK-3β-inhibitor, and MO-dot1l + GSK-3β-inhibitor electroporated retinas. Activation of Wnt signaling rescued the decrease in the number of proliferating progenitors upon dot1l knockdown after 51 h of intense light lesioning. Data represent means ± SEMs; n = 9–11 fish. ∗p < 0.05, p = 0.0167 (MO-ctl + DMSO versus MO-dot1l + DMSO) by 1-way ANOVA with Dunnett’s multiple comparisons test. (D) GSK-3β-inhibitor alone, Dot1l inhibitor (iDot1l) alone, or the combination was injected into the left eyes of Tg(1016tuba1a:GFP)zebrafish; DMSO alone was used as a control. At 51 h post-injection, eyes were collected for PCNA immunostaining. GSK-3β-inhibitor alone induced MG proliferation in undamaged eyes while co-injection of the Dot1l inhibitor led to no significant changes in number of proliferating MG. Data represent means ± SEMs. Each data point represents an individual fish. ∗∗∗∗p < 0.0001, by 1-way ANOVA with Dunnett’s multiple comparisons test. (E) Control MO or miR-216a MO was injected and electroporated into the left eyes of Tg(1016tuba1a:GFP)zebrafish, followed by either 4% DMSO or XAV939 (10 μM). Eyes were collected at 51 h post-injection and immunostained using antibodies against GFP for dedifferentiated MG and PCNA for proliferating progenitors. Nuclei were counterstained with TOPRO (blue). (F) Suppression of miR-216a by MO-216a injection stimulates MG proliferation; however, upon co-injection with XAV939, no significant increase in the number of proliferating progenitors was detected. (G) Quantification of PCNA+ proliferating progenitors in MO-ctl + DMSO, MO-216a + DMSO, MO-ctl + XAV939, and MO-216a + XAV939 electroporated retinas. Inhibition of Wnt signaling reversed the increase in the number of progenitors upon miR-216a knockdown after 51 h of intense light lesioning. Data represent means ± SEMs; n = 18–21 fish. ∗∗p < 0.01, p = 0.0089 (MO-ctl + DMSO versus MO-216 + DMSO) by 1-way ANOVA with Dunnett’s multiple comparisons test. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars, 50 μm. See also Figure S4. |
|
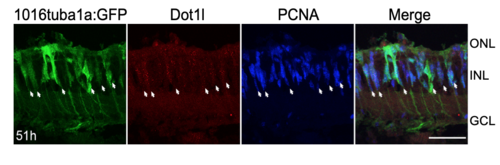
Dot1l is expressed in proliferating MG after photoreceptor loss. Related to Figure 3. Dot1l (red), GFP, and PCNA (blue) immunostaining after 51 hr intense light lesioning in Tg(1016tuba1a:gfp) retinas. Dot1l is expressed in proliferating MG. Arrows indicate GFP+/PCNA+ dedifferentiated MG that express Dot1l. Scale bar 50um. |
|
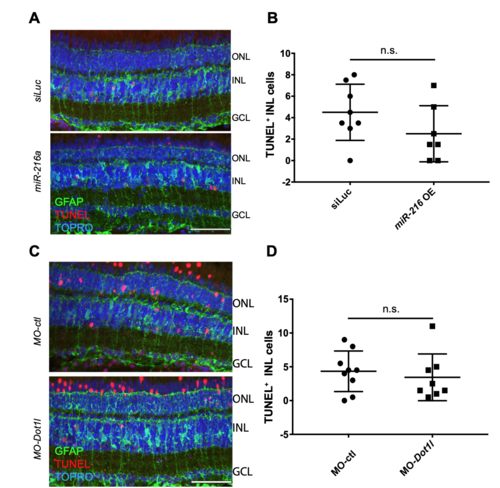
miR-216a mimic and Dot1l morpholino injections do not lead to increased apoptosis in the retina. Related to Figure 1 and Figure 4. (A) Control miRNA or miR-216a or (C) control morpholinos or Dot1l morpholinos were injected and electroporated into the left eyes of Tg(gfap:GFP) zebrafish before intense light exposure (0h). After 45h, retinas were collected, sectioned and TUNEL labeling was performed along with anti-GFP immunostaining. Nuclei were counterstained with TOPRO (blue). (B, D) TUNEL-positive cells in the INL were quantified. Data represent mean +/- SEM, n=7-9 fish. Two-tailed, Mann–Whitney U test is performed. ONL, Outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar 50um. |
|
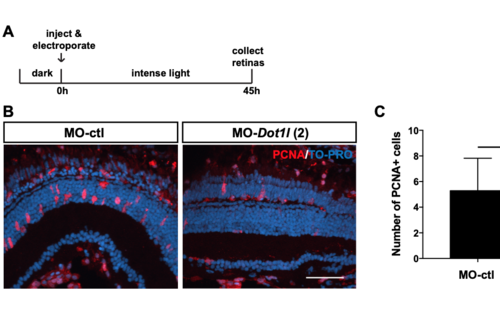
dot1l knock-down by a second independent morpholino injection inhibits MG proliferation during retina regeneration. Related to Figure 4. (A) Control morpholinos or a second independent dot1l morpholino (dot1l-MO-2) were injected and electroporated into the left eyes of Tg(1016tuba1a:gfp) zebrafish before intense light exposure (0h). After 45h, retinas were collected, sectioned and immunostained using antibodies against GFP, PCNA. Nuclei were counterstained with TOPRO (blue). (B) dot1l loss-of-function reduced the number of INL PCNA+ proliferating cells. (C) Quantification of PCNA+ proliferating progenitors in MO-ctl and MO-dot1l electroporated retinas. Data represent mean +/- SEM, n= 7-10 fish; *, p<0.05 by two- tailed Student’s t-test. ONL, Outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar 50um. |
|
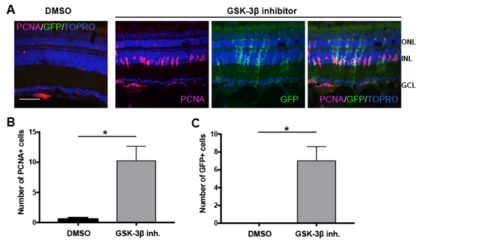
β-catenin stabilization stimulates MG dedifferentiation and proliferation. Related to Figure 7. (A) Tg(1016tuba1a:gfp) adult fish were intravitreally injected with 1mM GSK-3β inhibitor (n=8) or control vehicle (DMSO) (n=4). Eyes were collected 51h post injection and sectioned retinas were immunostained using antibodies against GFP for dedifferentiated MG and PCNA for proliferating progenitors. Nuclei were counterstained with TOPRO (blue). (B) Quantification of PCNA+ proliferating progenitors and (C) GFP+ dedifferentiated MG in control vehicle and GSK-3β inhibitor injected retinas. Data represent mean +/- SEM, n= 4-8 fish; *, p<0.05 by two-tailed, Mann–Whitney U test. ONL, Outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar 50um. |