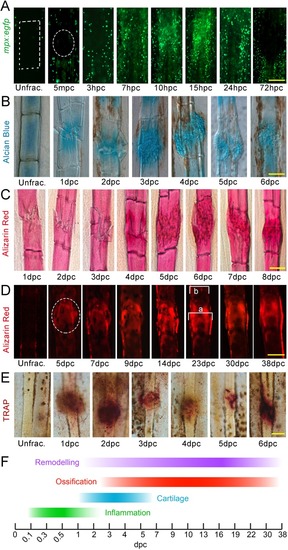
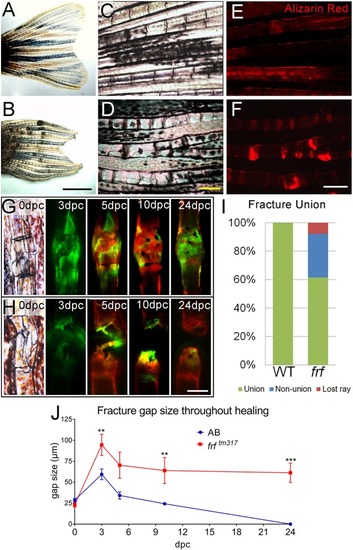
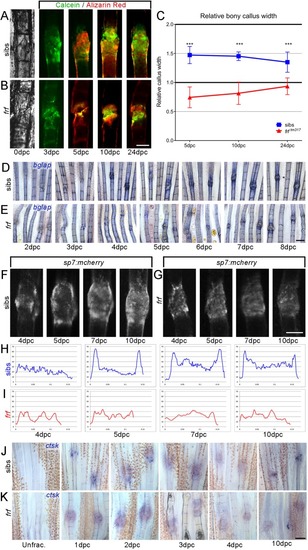
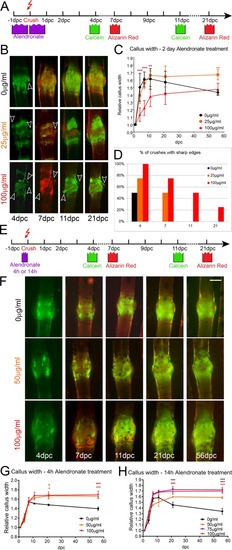
- Title
-
Clinical pathologies of bone fracture modelled in zebrafish
- Authors
- Tomecka, M.J., Ethiraj, L.P., Sánchez, L.M., Roehl, H.H., Carney, T.J.
- Source
- Full text @ Dis. Model. Mech.
|
|
|
PHENOTYPE:
|
|
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|