- Title
-
Knockdown of Laminin gamma-3 (Lamc3) impairs motoneuron guidance in the zebrafish embryo
- Authors
- Eve, A.M.J., Smith, J.C.
- Source
- Full text @ Wellcome Open Res
|
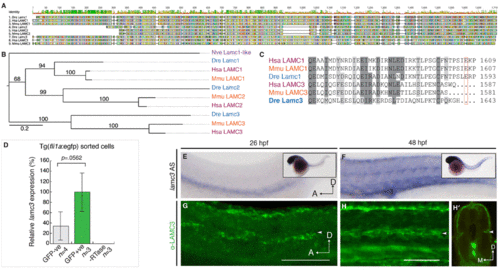
Lamc3 expression during zebrafish development. (A) MUSCLE-aligned protein “fingerprints” of zebrafish (Danio rerio, Dre), human (Homo sapiens, Hsa), and mouse (Mus musculus, Mmu) LAMC1, LAMC2, and LAMC3 amino acid sequences with RasMol colouring. (B) Unrooted RaxML phylogenetic tree (JTT+Gamma model) of Nematostella vectensis (purple, Nve) Lamc1-like and human (red, Hsa), mouse (orange, Mmu), and zebrafish (blue, Dre) LAMC1, LAMC2, and LAMC3 proteins. Branch labels denote ML bootstrap values (100). (C) Aligned human, mouse, and zebrafish amino acid sequence of the laminin γ1 and γ3 C-terminal tails show that the loss of the essential glutamic acid residue in γ3 is conserved across vertebrates. (D) qRT-PCR shows lamc3 is not significantly enriched in gfp+ endothelial cells (1.85E-05±5.49E-06, n=3) compared to GFP- cells (6.28E-06±1.48E-06, n=4) sorted from 26 hpf Tg(fli1a:egfp) embryos. Expression is relative to ef1α expression and normalised to GFP+ population (100%). All val |
|
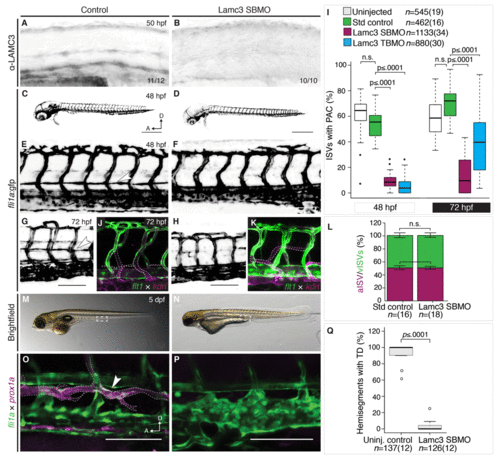
Lamc3 knockdown embryos do not develop the parachordal chain (PAC). (A–B) Lateral view of the zebrafish trunk stained using anti-LAMC3 antibody shows reduction in Lamc3 protein at 50 hpf in knockdown embryos (E, n=10) compared to standard controls (D, n=11). (C–I) Vasculature of 48 hpf Tg(fli1a:egfp) embryos. (C) Embryos injected with standard control MO develop the PAC. (D) Lamc3 knockdown embryos do not develop the PAC. (E) An enlarged image of the trunk vasculature in control embryos the PAC is indicated by a white arrowhead. (F) An enlarged image of the trunk vasculature in Lamc3 SBMO injected embryos. (G) The parachordal chain in control embryos at 72 hpf (white arrowhead). (H) By 72 hpf Lamc3 embryos have still not developed a parachordal chain. (I) Quantification of all ISVs with a PAC in Tg(fli1a:egfp) uninjected (white, n=545), control (green, n=462), Lamc3 SBMO knockdown (magenta, n=1133) and Lamc3 TBMO knockdown embryos (blue, n=880) at 48 hpf and 72 hpf, represent PHENOTYPE:
|
|
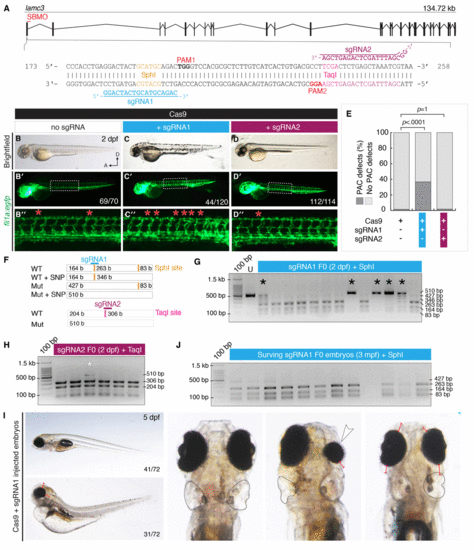
CRISPR/Cas9 mutagenesis of lamc3 causes defects in parachordal chain (PAC) development. (A) Design of sgRNAs targeted to exon1 of the lamc3 gene. Each sgRNA targets a restriction endonuclease site used for genotyping and is upstream of a protospacer adjustment motif (PAM). (B–D) Cas9/sgRNA injected embryos (as labelled) at 50 hpf. (B–D) Brightfield images show no developmental defects or developmental delay. (B′–D′) Whole embryo images of Tg(fli1a:egfp) fluorescence area indicated by white dotted line is enlarged in (B′′–D′′) the PAC in each hemisegment is indicated with a red asterisk. (E) Quantification of Tg(fli1a:egfp) embryos at 50 hpf with PAC defects. P-values determined by Fisher’s exact Two-tailed test compared to Cas9 alone controls. (F–I) 2% agarose genotyping gels with 100 bp ladder. (F) Schematic diagram of the region amplified by PCR contains two SphI sites (orange), which would digest into three fragments of 83, 164 and 263 bp in length. Predicted mutation of o |
|
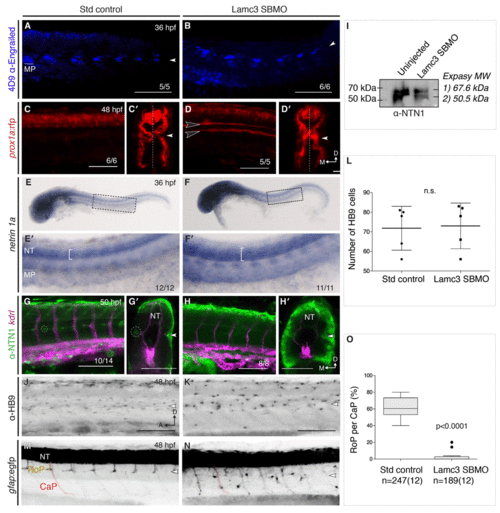
Lamc3 knockdown affects rostral primary motoneuron (RoP) migration. (A–B) Lateral view of 36 hpf embryos stained for muscle pioneers (MPs, underlined) using 4D9 anti-engrailed show MPs present in both control (A, n=5) and knockdown embryos (B, n=6). (C–D) 48 hpf Tg(prox1a:rfp) embryos injected with standard control (C, n=6) or Lamc3 SBMO (D, n=6) have increased prox1a expression in the trunk. Transverse sections in E′ and D′ show additional prox1a expressing cells at the horizontal myoseptum in Lamc3 SBMO embryos. (E–F) Whole-mount in situ hybridisation for netrin-1a expression at 36 hpf. Netrin-1a is expressed in MPs and neural tube (NT) in both control (E, n=12) and knockdown embryos (F, n=11). Highlighted regions are enlarged in E′ and F′ and indicate an increased expression domain in the NT of knockdown embryos. (G–H) Lateral view of the trunk of 50 hpf Tg(kdrl:mCherry) (magenta) embryos stained for Netrin-1 (anti-NETRIN-1, green). Loss of Netrin-1 at the horizontal myose |
|
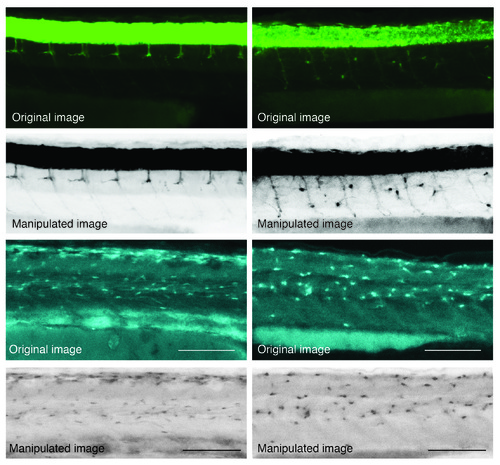
Examples of image manipulation from raw data (original images) as labelled. |
|
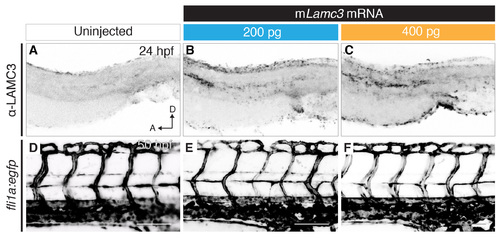
Overexpression of mouse Lamc3 mRNA. (A–C) Anti-LAMC3 immunostaining in the dissected trunks of 24 hpf embryos either uninjected (A), injected with 1 nl of 200 ng/μl of mouse Lamc3 (mLamc3) mRNA (B) or injected with 1 nl of 400 ng/μl of mouse Lamc3 mRNA shows increase of antibody staining at the horizontal myoseptum, dorsal plate of the neural tube and cloaca. (D–F) Imaging of the trunk vasculature in Tg(fli1a:egfp) 50 hpf embryos either uninjected (D), injected with 1 nl of 200 ng/μl of mouse Lamc3 mRNA (E) or injected with 1 nl of 400 ng/μl of mouse Lamc3 mRNA (F). A, anterior; D, dorsal; hpf, hours post fertilisation. Scale bars: 100 μm. |
|
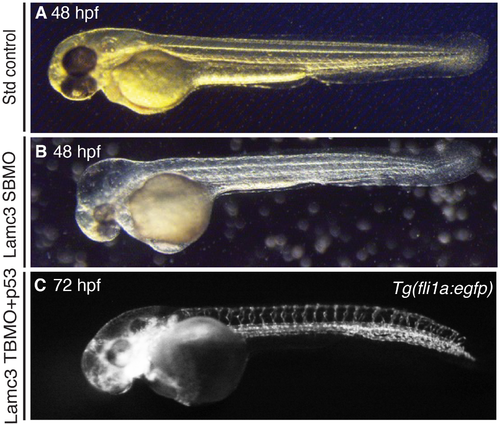
p53 and Lamc3 translation blocking morpholino (TBMO) injections. (A) 48 hours post fertilisation (hpf) embryo injected with standard control morpholino. (B) 48 hpf embryo injected with Lamc3 splice-blocking morpholino (SBMO) without p53 morpholino causes reduced eye size and oedema in the head. (C) Tg(fli1a:egfp) embryo at 72 hpf injected with Lamc3 TBMO and p53 morpholino shows loss of the parachordal chain. |
|
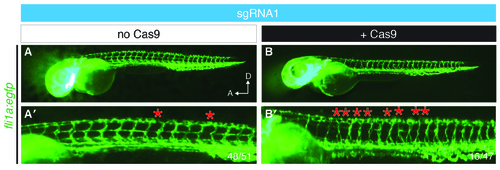
sgRNA injection does not affect parachordal chain (PAC) development. (A) In 50 hpf Tg(fli1a:egfp) embryos injected with 1nl of 12–15 ng/μl of sgRNA1 failure of PAC development (red asterisk) is observed in fewer than 5 hemisegments at the yolk extension per embryo (48/51). (B) A third of embryos injected with sgRNA1 and Cas9 mRNA have perturbed development of the PAC in 5 or more hemisegments at the level of the yolk extension (16/47). A, anterior; D, dorsal; hpf, hours post fertilisation. |