- Title
-
Rpgrip1 is required for rod outer segment development and ciliary protein trafficking in zebrafish
- Authors
- Raghupathy, R.K., Zhang, X., Liu, F., Alhasani, R.H., Biswas, L., Akhtar, S., Pan, L., Moens, C.B., Li, W., Liu, M., Kennedy, B.N., Shu, X.
- Source
- Full text @ Sci. Rep.
|
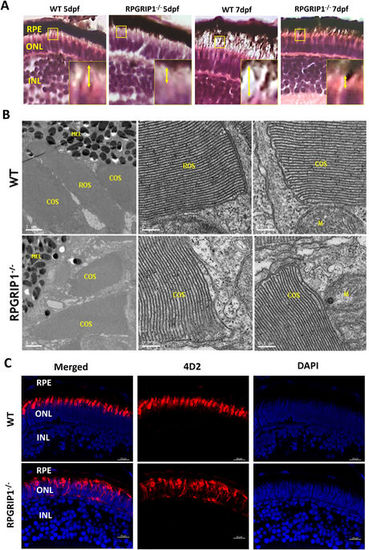
Rod outer segments were not developed in rpgrip1 −/− zebrafish. (A) Photoreceptor outer segments and inner segments were shorter in the retinas of rpgrip1 −/− zebrafish at 5 and 7dpf shown by haematoxylin & eosin staining. Double-headed yellow arrow shows the outer/inner segments; the measurement of outer segment length was shown in Supplementary Materials Fig. S3. Inserts show a 3.5 × magnification of the selected regions. (B) Ultrastructural analysis using transmission electron microscopy revealed both rod and cone outer segments were formed in the retinas of 5dpf wildtype siblings but only cone outer segments were presented in the mutants; rod outer segments were not observed in the retinas of 5 examined RPGRIP1 −/− mutants at 5dpf. (C) Immunostaining with anti-rhodopsin antibody (4D2) further confirmed rod outer segments were not formed in the retinas of rpgrip1 −/− mutants at 5dpf; rhodopsin was mislocalized in whole rod cell body. Nuclei were shown in blue using DAPI. COS, cone outer segments; GCL, ganglion cell layer; INL, inner nuclear layer; M, mitochondria; MEL, melanosome; ONL, outer nuclear layer; RPE, retinal pigment epithelium; ROS, rod outer segments. EXPRESSION / LABELING:
PHENOTYPE:
|
|
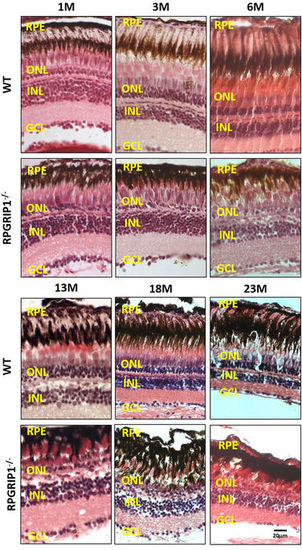
Haematoxylin & eosin staining of retinal sections of wild type and rpgrip1 −/− zebrafish at different ages, showing progressive retinal degeneration. WT, wildtype; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. The scale bars are 10 µm. PHENOTYPE:
|
|
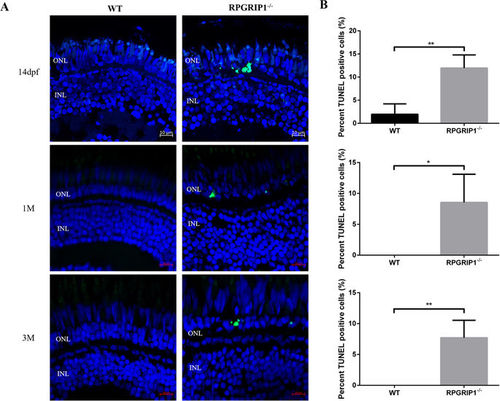
TUNEL assay showed significantly increased cell death in rpgrip1 −/− mutant retinas. Retina cryosections of wildtype (WT) and rpgrip1 −/− zebrafish at 14 dpf, 1 mf and 3 mpf were subjected to TUNEL assay. (A) TUNEL-positive cells were present in the outer nuclear layer (ONL) of rpgrip1 −/− mutant retinas. TUNEL-positive cells were barely observed in ONL of wildtype retina at three age points. (B) Graph indicating significant cell death (12%, 9% and 8% respectively) in the mutant retina compared to the wildtype retina at each age point. The data were collected by counting positive cells from 5 retinal sections of wildtype and rpgrip1 mutant respectively and shown as mean ± SD. SD: standard deviation. **p < 0.01. PHENOTYPE:
|
|
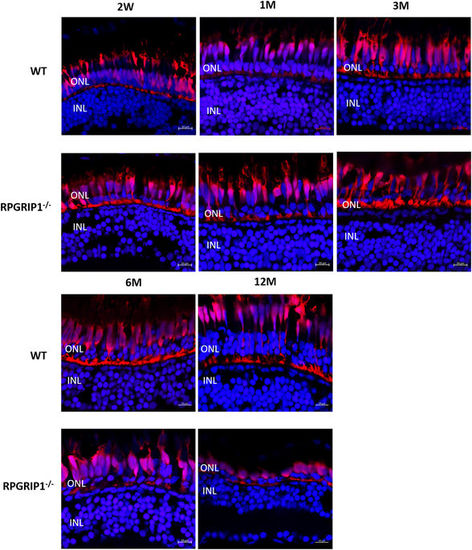
Immunostaining with Zpr1 antibody exhibited red/green double cones that were normal at early ages and degenerated at later ages. Nuclei were shown in blue using DAPI. Double cones of rpgrip1 −/− mutants were morphologically normal at 2wpf and 1 mpf, shown here. While at 3mpf, double cones were disorganized, the number of double cones was markedly reduced at 6 mpf and only some double cone cell bodies remained at 12 mpf. EXPRESSION / LABELING:
PHENOTYPE:
|
|
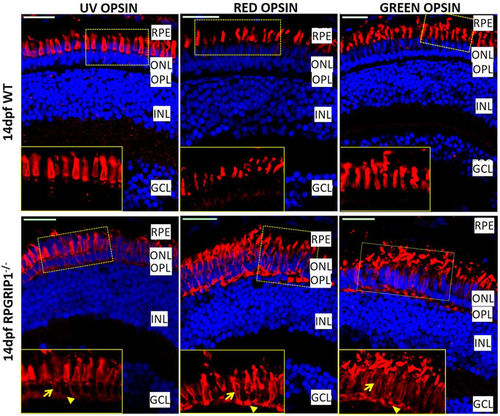
Partial mislocalization of cone opsins in rpgrip1 −/− retinas. Green, red and UV opsins were localized in cone outer segments of 14 dpf wildtype zebrafish retinas. In rpgrip1 mutant zebrafish retinas, green, red and UV opsins were localized in outer segments and partially mislocalized in cone cell bodies (arrow) and synapses (arrowhead). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; OPL, outer plexiform layer; RPE, retinal pigment epithelium. EXPRESSION / LABELING:
PHENOTYPE:
|
|
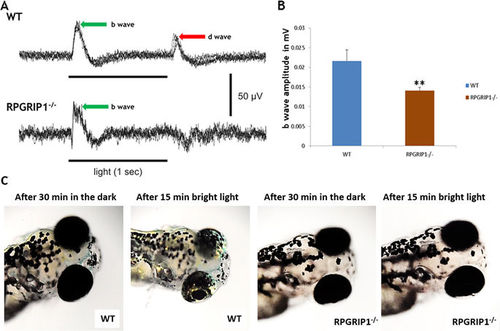
Impairment of visual function in rpgrip1 −/− zebrafish. (A) Representative ERG traces from wildtype and rpgrip1 −/− zebrafish at 7dpf. D-wave was absent in the mutant zebrafish. (B) b-wave amplitudes of rpgrip1 −/− zebrafish (n = 10) were significantly reduced when compared to wildtype siblings (n = 11). The result is shown as mean ± SD. SD: standard deviation. **p < 0.01. (C) Visual background adaption for wildtype (n = 10) and rpgrip1 −/− zebrafish (n = 10) at 7dpf responding to dark adaption and bright light stimulation. Representative images showing that melanin of wildtype zebrafish was aggregated into small granules after light adaption, while rpgrip1 −/− zebrafish displayed larger diffused melanin granules. PHENOTYPE:
|
|
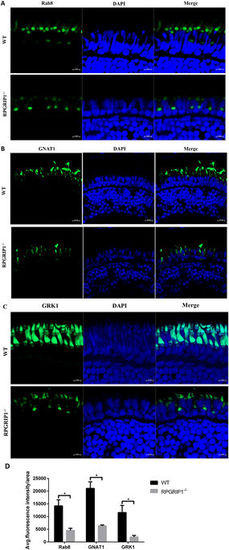
Mislocalization of ciliary proteins in rpgrip1 mutant fish. (A) Rab8 was mainly localized to the connecting cilium of rod cells and some weak fluorescence signals were present in the outer segments of photoreceptor cells in wildtype (WT) zebrafish at 2 wpf; it was mislocalized in inner segments of photoreceptor cells and the localization to the outer segments of photoreceptor cells was lost in rpgrip1 mutant zebrafish at 2 wpf. (B,C) Transducin and GRK1 exhibited abnormal distribution in cone outer segments and loss of localization to rod outer segment, because the rod outer segments were absent in rpgrip1 mutant zebrafish. INL, inner nuclear layer; ONL, outer nuclear layer. (D) Quantification of fluorescence intensity of Rab8, GNAT1 and GRK1 signals. The fluorescence intensity was measured within three outer segment areas (25 × 25 µm²/area) in each section (four sections from four individual retinas were used) by Zen (Zeiss) at the condition of same laser intensity and master gain, and the data was analysed by t test with Graph Prism. The result is shown as mean ± SD. SD: standard deviation. p = 0.0027 (Rab8); p = 0.0084 (GNAT1); p = 0.0164 (GRK1). EXPRESSION / LABELING:
PHENOTYPE:
|
|
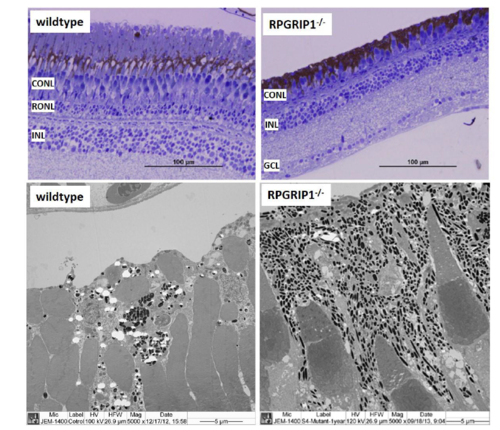
Retinal structure of wildtype and rpgrip1-/- zebrafish at age of 13 months showed only short cones remained in rpgrip1-/- zebrafish retina. Upper panel, light microscopy structure of wildtype and rpgrip1-/- zebrafish retinas; lower panel, ultrastructure of wildtype and rpgrip1-/- zebrafish retina. CONL, cone outer nuclear layer; GCL, ganglion cell layer; INL, inner nuclear layer; RONL, rod outer nuclear layer. |
|
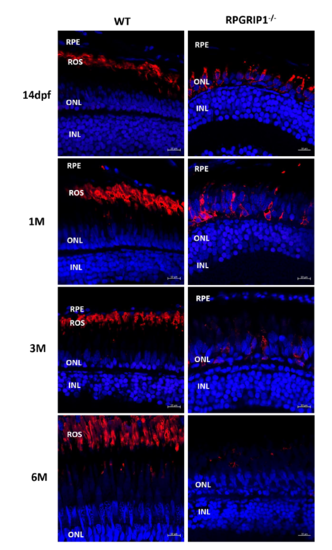
Immunostaining of retinal sections from wildtype and rpgrip1-/- zebrafish at age of 14 days (dpf), one month (1 mpf), 3 months (3 mpf) and 6 months (6 mpf). 4D2, labelling rhodopsin, was used for the staining. Nuclei were shown in blue using DAPI. Rhodopsin was mislocalized in all examined rpgrip1-/- mutant retina. The signal of rhodopsin was significantly decreased at 1mpf, almost absent at 3mpf, and at background level at 6 mpf. INL, inner nuclear layer; ONL, outer nuclear layer; ROS, rod outer segment; RPE, retinal pigment epithelium |
|
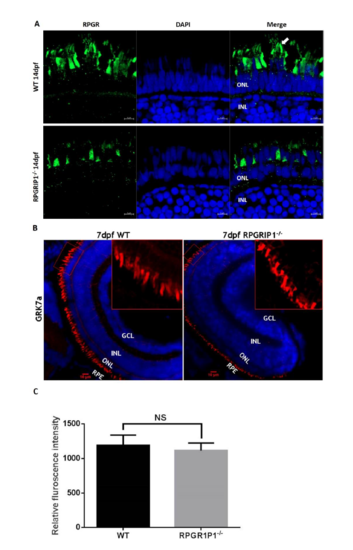
Immunostaining of retinal sections of wildtype and rpgrip1-/- zebrafish using anti-RPGR antibody showed abnormal localization of Rpgr. Rpgr was localized to the connecting cilia and outer segments of photoreceptors in 14dpf wildtype zebrafish retina, while Rpgr was mislocalized in inner segments of photoreceptor cells of rpgrip1-/- mutant retinas and the fluorescence signals were significantly decreased. Arrow shows rod outer segment. (B) There is no difference in GRK7a localization to the cone outer segments in both wildtype and rpgrip1 mutant zebrafish at 5dpf. (C)The fluorescence signals of the boxed areas of wildtype and mutant retinal sections were measured using Image J software. There is no significant difference between wildtyep and mutants with p=0.6989. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. NS, no significance. EXPRESSION / LABELING:
PHENOTYPE:
|