- Title
-
Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype
- Authors
- Fraser, B., Duval, M.G., Wang, H., and Allison, W.T.
- Source
- Full text @ PLoS One
|
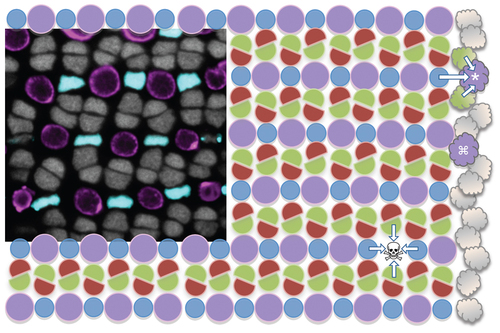
Zebrafish cone photoreceptor mosaic and experimental rationale. The cone photoreceptor mosaic of zebrafish consists of a precise reiterated arrangement of cone spectral sensitivity subtypes, extending in rows radiating toward the periphery of the retina (towards the right). The inset micrograph demonstrates the positions of the UV- and blue-sensitive cones (pseudocoloured magenta and blue, respectively, in a transgenic fish with these cone subtypes expressing GFP and mCherry), and the nuclei of green- and red-sensitive cones forming rows in between (grey). This tangential view of the cones is orthogonal compared to Figure 2, at a level just above the magenta BrdU+ nuclei in panel 2E. This mosaic is schematized, with magenta circles representing the UV cones and other coloured shapes representing their respective cone sensitivities. New cones are continuously added to the periphery of the adult retina from stem cells near the iris (cloud shapes on right); thus the existing mosaic serves as a template for specifying cone identity and/or position of newly added retina. Towards the top right one can imagine a UV cone (*) during differentiation, and its specification being influenced by the identity of the existing mosaic (arrows). Alternatively, mathematical modelling also supports that newly generated cones could be specified without regard for position (e.g. differentiating UV cone marked with adjacent to an existing UV cone, this arrangement is not observed in mature mosaic) and subsequently move to their correct position. Our intervention principally ablates UV cones (skull & cross-bones, ) and hypothesizes that the fate of the regenerating cell will be influenced (arrows) by the remaining cones. |
|
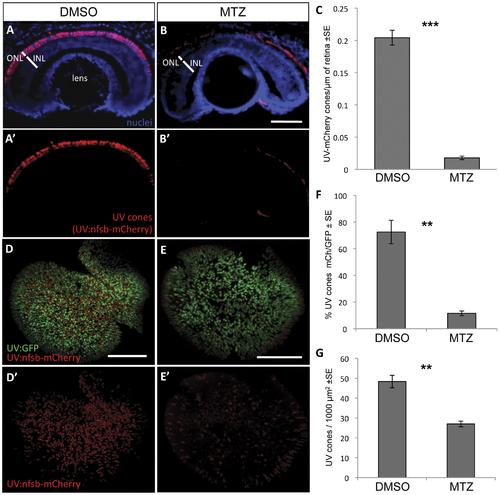
UV cone photoreceptor ablation by prodrug application to transgenic fish. Fish were engineered with inducible cell ablation transgenes expressed in UV cone photoreceptors, Tg(SWS1:Gal4-VP16)ua3016;Tg(UAS-E1b:NfsB-mCherry)c264 (“UV:nfsb-mCherry”). Fish treated with a vehicle control DMSO solution maintained nitroreductase- (nfsb-) mCherry expression in UV cones and cell death was not induced (A, A′). Siblings of these fish, treated with prodrug metronidazole (MTZ) for 48 hrs, lost the mCherry fluorescence due to ablation of the targeted UV cones (B, B′). Note the red fluorescence in panels B and B′ is auto-fluorescence due to a longer exposure compared to panel A. Quantification of UV cones in these cryosections after treatment with the prodrug MTZ (C) revealed a significant decrease in the number of cones expressing mCherry fluorescence in the ONL compared to vehicle-treated controls (***p<0.0001; DMSO treated n = 10, MTZ treated n = 8). Similar observations were made on flat-mounted retina (D,E) wherein the UV cones expressing nfsb-mCherry decreased in abundance relative to the number of UV cones expressing GFP (F, **p<0.001; n = 9 DMSO-treated, n = 10 MTZ-treated) or when considering the absolute density of all UV cones per unit area (G, **p<0.001). Scale bar = 50 µm in A,B and 100 µm in D,E. |
|
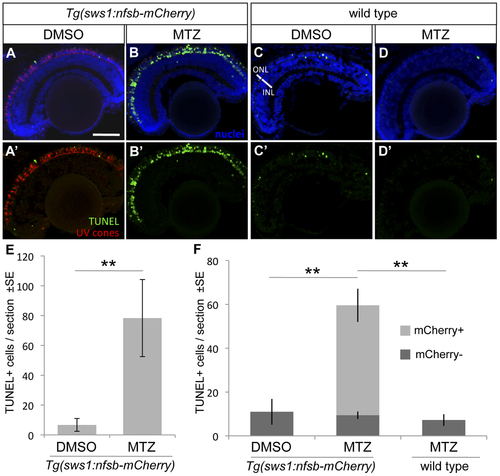
UV cone photoreceptor death induced by prodrug application to transgenic fish. An abundance of apoptotic cells were detected in the ONL of the retina of Tg(SWS1:Gal4-VP16)ua3016;Tg(UAS-E1b:NfsB-mCherry)c264 fish treated with MTZ for 48 hrs (B, B′) compared to various controls. Very little apoptosis was found in the ONL of Tg(SWS1:Gal4-VP16)ua3016;Tg(UAS-E1b:NfsB-mCherry)c264 fish treated with a control DMSO solution (A, A′). Non-transgenic siblings treated with either DMSO or MTZ (C or D, respectively) for 48 hrs also possessed few apoptotic cells. E. The number of TUNEL+ cells in retinal sections equivalent to A and B were quantified, showing an order of magnitude increase upon MTZ prodrug application (**p<0.001, MTZ-treated n = 11, DMSO treated n = 15). F. A repetition of this labelling to discern the bystander effect, i.e. compare the number of TUNEL+ cells that do not express nfsb-mCherry as compared to basal levels in normally developing fish. Again the total number of TUNEL+ cells is increased when the transgenic fish are treated with prodrug MTZ rather than DMSO vehicle control (compare total height of light+dark bars, MTZ-treated n = 7). This contrasts the abundance of TUNEL+ cells without mCherry, which is not increased relative to DMSO control fish nor to wildtype fish receiving MTZ (dark grey bars, p = 0.84 and p = 0.927, respectively wherein n = 4 or 3, with means comparable to panel E). Scale bar = 50 µm. |
|
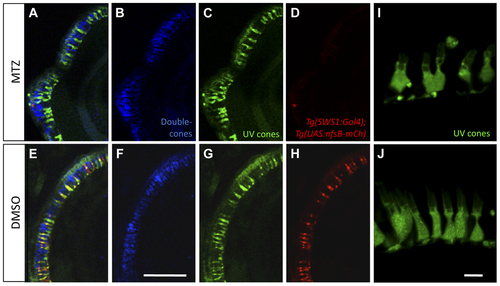
Morphological analysis of photoreceptors indicates that metronidazole does not damage neighbouring cells. UV cones expressing nitroreductase (NTR) were ablated after the addition of prodrug metronidazole (MTZ) (A–D), while nfsB-mCherry persisted in transgenic fish treated with DMSO vehicle control (E–H). 24 hours after ablation, UV cones lacking NTR-expression appeared morphologically normal (C) based on their GFP expression compared to controls (G). Red-green double cone pairs were detected with the antibody zpr1. Zpr1 labelling was consistent following MTZ ablation (B) showing normal cell morphology compared to the controls (F). Scale bar = 25 µm. A magnified view of remaining UV cones (I) with normal morphology comparable to controls is presented from a separate sample (J, Scale bar = 5 µm). |
|
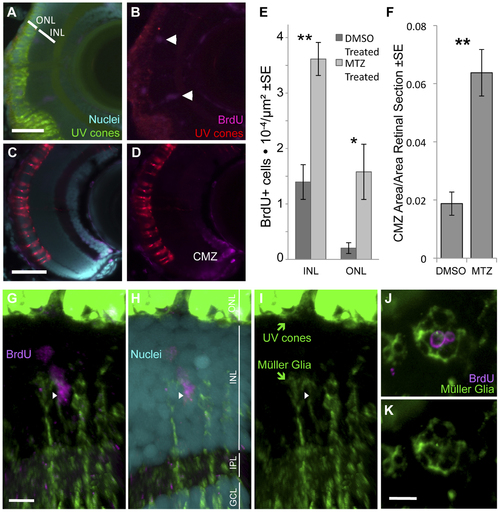
Ablation of cones, primarily UV cones, is Our transgenic UV cone ablation model fish treated with prodrug MTZ showed a significant increase in proliferating cells in the retina at 24 hours after ablation (A, B, especially notice cells demarcated with arrowheads in top right away, from the retinal margin (ciliary marginal zone, CMZ)) compared to sibling fish treated with a DMSO vehicle control solution (C, D). BrdU is incorporated into the CMZ of all fish with and without cone ablation, as expected (A–D). Proliferating cells were quantified in the inner and outer nuclear layers (INL and ONL) at 24 hours after ablation (E) (**INL p = 0.004, *ONL p = 0.002, DMSO treated n = 9, MTZ treated n = 10). The area of the CMZ was also greater in extent after MTZ treatment, calculated relative to the area of the entire retinal sections (F, **p<0.001, MTZ-treated n = 11, DMSO treated n = 15). Scale bars = 5 & 3 µm in A & C, respectively. G–I. The proliferating cells that increase in abundance during regeneration include Müller glia in the INL, as indicated by close apposition of Müller glia markers (green) with BrdU+ nuclei (magenta) (arrowhead). Example shown is from Movie S1 with a cocktail of two antibodies against Müller glia (green, anti-GFAP & anti-glutamine synthetase, both raised in mouse. Saturated green at top of figure is from UV cones expressing abundant GFP, scale bar = 5 µm) and anti-glutamine synthetase antibody produced similar results alone (Movie S2). ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. J–K. Same labelling regimes as panel G but as a tangential section (orthogonal to G and equivalent plane to Figure 1), showing BrdU+ nuclei in the INL surrounded by Müller glia. Scale bar = 5 µm. |
|
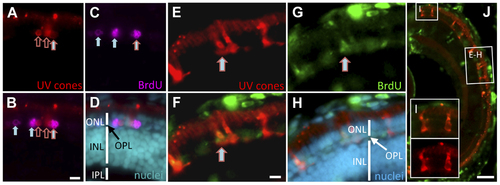
Regeneration of UV cones following prodrug cell ablation. Regenerating UV cones were observed in larvae 5 days after MTZ treatment. Co-localization of these UV cone markers with BrdU detection indicated that the UV cones were recently proliferating. The right-most cell (A–C) is double labelled for BrdU and UV opsin (mCherry) (filled arrow with red outline; has not yet differentiated to a cone morphology). Other UV cones have begun to reappear (empty red arrows), presumably outside of the window of BrdU application. Other BrdU-positive photoreceptors are detectable (filled arrows), likely representing rods, nascent UV cones, or UV cones not expressing the transgene. One week after MTZ ablation, the regeneration of morphologically mature cones was observed (E–H). BrdU+ cells co-labelled with UV cone mCherry expression (E–G; arrow). The regenerated UV cones are morphologically similar to newly generated UV cones in the expanding retinal margin (I), demonstrating that the regenerated cones are qualitatively normal. Scale bars for A–H = 5 µm, J = 50 µm. EXPRESSION / LABELING:
|
|
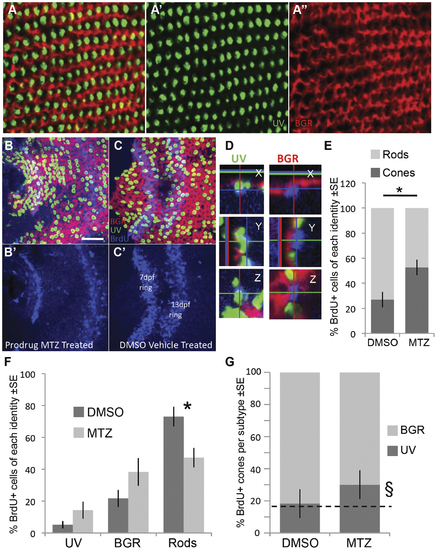
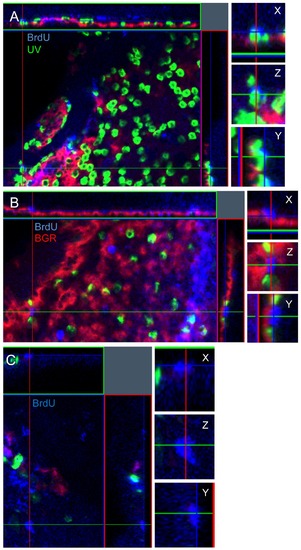
Following ablation of UV cones, the regenerating photoreceptors were more likely to become cones. Opsin riboprobe labelling to quantify the identity of regenerating cones: Throughout this figure, UV opsin riboprobe is green, and a cocktail of blue-, green- & red-opsin riboprobes are labelled in red. A. Post-larval areas of adult retina have the expected row mosaic pattern (see Figure 1) A′ & A′′ present individual riboprobes merged in A. B,C. Retinas labelled and oriented as per panel A (see also Figure 1), with BrdU located near the less-well-organized optic nerve head. Two rings of BrdU are visible marking cells that were dividing at the retinal periphery during BrdU application (7 & 13 days post fertilization, dpf, also shown separately in B′,C′). Only BrdU+ cells located between the two rings or inner to the 7 dpf ring were analyzed for co-localization with a photoreceptor subtype marker. Panel B is retina that had UV cones ablated by application of prodrug, panel C is control retina receiving vehicle control only. D. Magnified view of BrdU+ cells (blue) in three dimensions from images equivalent to panel B; images in panel B are presented in the ‘Z view’. Cell in the left column was categorized as a UV cone because the UV opsin riboprobe label (green) is contiguous with the apical side of the BrdU+ nucleus. Cell in the right column was classified as a BGR (Blue, Green or Red cone) because the cocktail of these opsin riboprobes labels (red fluorescence) an area contiguous with the apical side of the BrdU+ nucleus. Apical is up in the X view, and to the left in Y view. E. As expected, most BrdU+ cells in control retina are rods, contrasting results following UV cone ablation by MTZ application, where the majority of BrdU cells are cones (* p = 0.008 by Kruskal-Wallis analysis of variance, N = 6 fish each in MTZ and DMSO vehicle control, equally divided amongst two trials). F. The number of BrdU+ cells per photoreceptor subtype (sub-dividing the results in panel E). Normal control retinas mostly generated rods, and the proportion of cone subtypes is coordinately but non-significantly increased. G. The relative abundance of BrdU+ UV cones, compared to other BrdU+ cone subtypes, did not increase significantly (p = 0.064) as assessed by a non-parametric analysis of variance per fish (N = 6). The proportion of UV cones matched the expected frequency of 16.7% (dotted line) in DMSO vehicle control treated fish (18.3%, χ2 p = 0.769, n = 84 BrdU+ cones), but was higher than the expected frequency following UV cone ablation when assessed as a sum of all fish (§, 30.1%, χ2 p = 0.0002, n = 311 BrdU+ cones in 6 fish). Overall, cone genesis increased following cone ablation, and future work is needed to determine the extent to which UV cones are more likely to regenerate relative to other subtypes following UV cone ablation. EXPRESSION / LABELING:
|
|
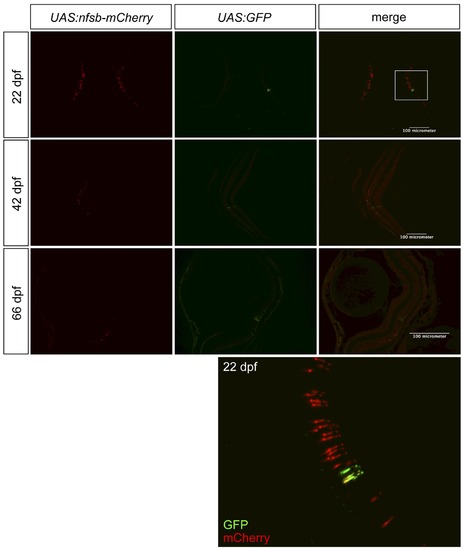
Assessing the quality of transgene expression in photoreceptors. We assessed our novel transgenic driver line, intended to express Gal4-VP16 in UV cone photoreceptors, by breeding it to two reporter lines. One reporter, primarily used in our experiments, drives expression of nfsB-mCherry (NTR-mCherry protein), and the other reporter is GFP. Thus breeding created Tg(SWS1:Gal4-VP16)ua3016;Tg(UAS-E1b:NfsB-mCherry)c264;Tg(4xUAS:GFP)hzm3 zebrafish. The inset, from a fish at 22 days post-fertilization (dpf), is magnified at the bottom of the figure and is consistent with data from fish at 42 or 66 dpf, regarding GFP being present in more cones than mCherry. Thus we cannot rule out deficits with the Gal4-VP16 driver line as contributing to the lack of robust NTR-mCherry expression in all UV cones. Further, the quantity of nfsb-mCherry expressing cells is substantially decreased in older fish. |
|
Confocal z-stack analysis to determine the identity of BrdU-positive photoreceptors. A three-dimensional analysis was performed using the ZEN microimaging software to allow for the visualization of BrdU in the nucleus co-localizing with opsin expression. Photoreceptors were divided into 3 unambiguous categories: BrdU-positive co-localizing with UV opsin (A), BrdU-positive co-localizing with BGR opsin (B), and non-colocalizing BrdU-positive rods (C). The BrdU+ rod in C is located in the vitreal side of the ONL compared to panels A and B, thus cells towards the right of the panel lack cone opsin labelling. |