- Title
-
Postembryonic neuronal addition in Zebrafish dorsal root ganglia is regulated by Notch signaling
- Authors
- McGraw, H.F., Snelson, C.D., Prendergast, A., Suli, A., and Raible, D.W.
- Source
- Full text @ Neural Dev.
|
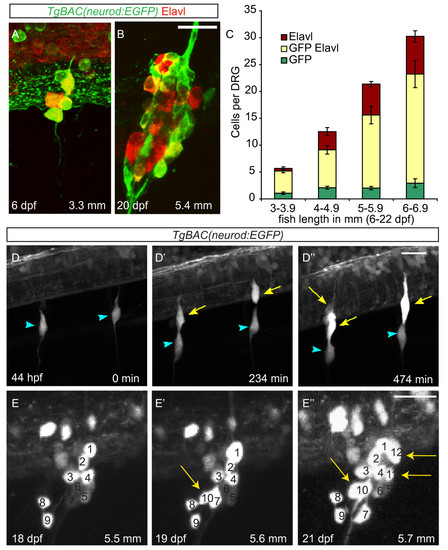
Neurons are continuously added to the larval dorsal root ganglia (DRG). (A-B) Confocal images of a TgBAC(neurod:EGFP) transgenic larva between six (A) and twenty-two dpf (B) showing DRG growth. Neurons were labeled with anti-Elavl (red) and GFP (green) antibodies. (C) Quantification (±SEM) of Elavl and GFP-positive cells per DRG in larvae staged by overall body length in millimeters (mm). Elavl-positive neurons are continuously added to the DRG as the larvae develop. Over half of the Elavl-labeled cells are also labeled with GFP (n = between five and fifteen larvae per condition, four ganglia per larva). (-D′′) Stills from a time-lapse movie showing neuron differentiation in a TgBAC(neurod:EGFP) embryo between 44 and 52 hpf. (D) At 44 hpf the DRG contain a single neuron each (blue arrowheads). (D′) By 234 minutes, neurons have been added to each ganglion (yellow arrows). (D′′) At 474 minutes the ganglia contain several neurons. (E-E′′) Analysis of a single ganglion in a TgBAC(neurod:EGFP) transgenic larval fish between 18 and 21 dpf. (E) At eighteen dpf, the ganglion contains nine GFP-positive cells. (E′) At 19 dpf, the ganglion contains 10 GFP-positive cells (white arrow) and by 21 dpf (E′′) 12 GFP-positive cells (white arrows). During the course of imaging the larval fish grew from 5.5 mm to 5.7 mm in length. Scale bars, 20 μm. dpf, days postfertilization; hpf, hours postfertilization; GFP, green fluorescent protein. |
|
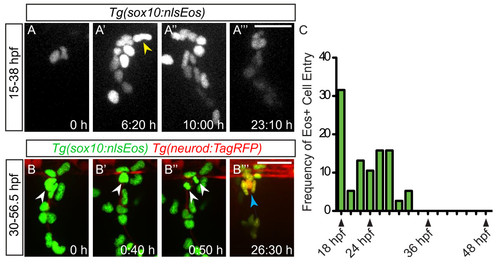
Neural crest migration into the dorsal root ganglia (DRG) ceases prior to neuron differentiation. (A-A′′ ′) Confocal projections of stills from a time-lapse movie showing migrating Tg(sox10:nlsEos) + neural crest cells between 15 and 38 dpf. (A) At time zero, the region of DRG formation contains two Eos + cells. (A′) A still at 6:20 hours shows a representative neural crest cell joining the DRG anlagen (yellow arrowhead). Between 10:00 hours (A′′) and 23:10 hours (A′′ ′), no neural crest cells migrate into the DRG. (B-B′′ ′) Time-lapse movie a Tg(sox10:nlsEos)/Tg(neurod:TagRFP) transgenic embryo between 30 and 57:10 hpf. (B) An Eos+/RFP- cell (white arrowhead) at time zero, begins to express RFP by 0:40 hours (B′) and divides at 0:50 hours (B′′). (B′′ ′) After 26.30 hours of imaging, neurons are labeled with RFP (blue arrow). (C) Quantification of Eos + cells that migrate into the DRG anlagen between 18 and 48 hpf. No new neural crest cells join the DRG after 32 hpf (n = five embryos). Scale bars, 25 μm. dpf, days postfertilization; hpf, hours postfertilization. |
|
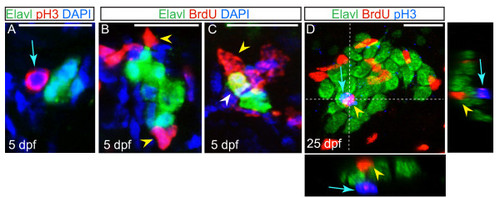
Non-neuronal cells associated with the dorsal root ganglia (DRG) proliferate. (A) Confocal projection of a ganglion at five dpf, showing sensory neurons labeled with Elavl (green) and an associated cell labeled with pH3 (red; blue arrow). Nuclei were labeled with DAPI (blue). (B-C) Ganglia of larvae at five dpf following BrdU incorporation (red). Neurons are labeled with Elavl (red) and nuclei are labeled with DAPI (blue). (B) Following a BrdU pulse at five dpf, cells associated with the DRG incorporate BrdU (yellow arrowhead), but neurons do not. (C) In a larva that was pulsed with BrdU at two dpf and chased to five dpf, a BrdU + neuron is located in the DRG (white arrowhead). (D) A ganglion in a 25 dpf, 6.25 mm larval fish exposed to a pulse of BrdU at 25 dpf, shows BrdU + cells (red; yellow arrowhead) and pH3 labeled cells (blue; blue arrow) are intermingled among Elavl + neurons (green), no neurons are labeled with BrdU or pH3. Dashed lines indicate levels of XZ and YX insets. Scale bars, 20 μm. BrdU, bromodeoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; dpf, days postfertilization. pH3, phosphohistone H3. |
|
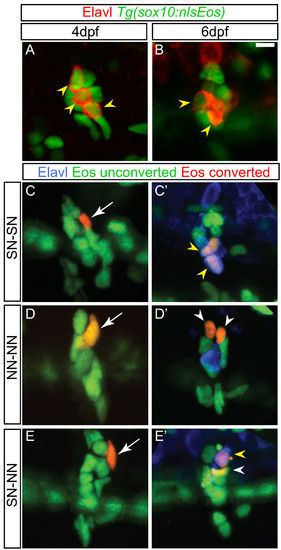
Dividing progenitors within the larval dorsal root ganglia (DRG). (A-B) Confocal projections of ganglia-containing cells that express the Tg(sox10:nlsEos) transgene (green) and neurons labeled with Elavl (red). At four dpf (A) and six dpf (B) Tg(sox10:nlsEos) labels both neurons (yellow arrowheads) and non-neuronal cells. (C-E)Tg(sox10:nlsEos) cells were photoconverted by brief exposure to 405 nm light at four dpf, resulting in red fluorescence (white arrow). (C′-E′) At six dpf, larvae were labeled with Elavl (blue) and analyzed by confocal microscopy. Shown are examples of cells that divided and gave rise to two sensory neurons (SN-SN; yellow arrowheads) (C-C′), two non-neuronal cells (NN-NN; white arrowheads) (D-D′) or one sensory neuron and one non-neuronal cell (E-E′). Scale bar, 20 μm. dpf, days postfertilization. |
|
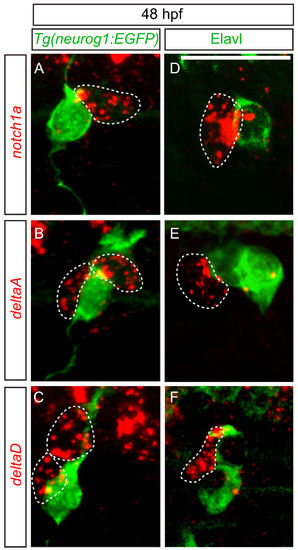
delta and notch mRNAs are expressed in cells associated with dorsal root ganglia (DRG) neurons. (A-C) Confocal images of notch1a, deltaA and deltaD fluorescent RNA in situ hybridization in 48 hpf Tg(neurog1:EGFP) transgenic embryos. (A) Expression of notch1a (red, dashed line) is found in a cell that neighbors GFP + DRG neuron (green). Both deltaA (B) and deltaD (C) are expressed in cells surrounding GFP + neurons. (-F) Comparison of anti-Elavl labeling and notch1a, deltaA and deltaD RNA expression at 48 hpf. (D) A cell expressing notch1a (red) is seen adjacent to an Elavl-positive neuron (green). (E) deltaA is expressed in cells surrounding Elavl-positive neurons, as is deltaD (F). Scale bars, 20 μm. hpf, hours postfertilization. |
|
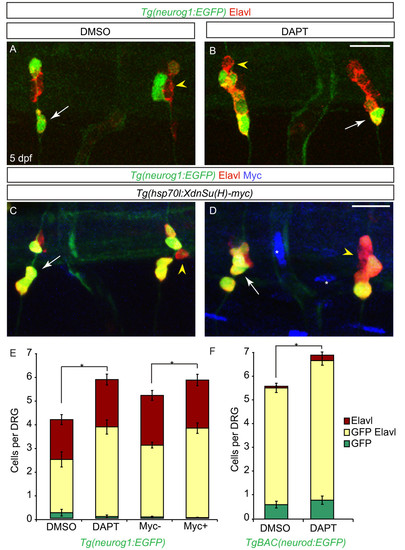
Notch inhibition during larval development leads to an increase in dorsal root ganglia (DRG) neurons. (A-B) Confocal projections of Tg(neurog1:EGFP) DRG neurons labeled with anti-GFP (green) and Elavl (red) from larvae treated with DMSO or DAPT between two and five dpf. At five dpf, DRG neurons are labeled by Elavl (yellow arrowhead) and a subset also express GFP (white arrow) in both DMSO (A) and DAPT (B) treated larvae. (C-D) Confocal images of Tg(hsp70l:XdnSu(H)myc); Tg(neurog1:EGFP) larvae following heat-shock (HS) beginning at two dpf. In control (C) and transgenic (D) larvae at five dpf after HS, DRG neurons were labeled with GFP (green) and Elavl (red). Larvae carrying the Tg(hsp70l:XdnSu(H)myc) transgene were identified by ectopic myc labeling (blue, white asterisk) following HS. (E) Quantification of DRG neurons following Notch inhibition with DAPT or HS in Tg(hsp70 DN (Su(H) myc) embryos from two to five dpf. There is a significant increase in the number of DRG neurons in DAPT-treated and myc-positive larvae as compared to control larvae. (n = 6 for DMSO- or DAPT-treated larvae, n = 10 for myc-negative larvae and n = 13 for myc-positive larvae, five ganglia per larvae, *P <0.03, Student’s t test). (F) Quantification of DRG neurons in TgBAC(neurod:EGFP)-positive DRG following Notch inhibition with DAPT from two to five dpf. There is a significant increase in the number of DAPT-positive DRG neurons as compared to control embryos. (n = 9 for DMSO or n = 8 for DAPT-treated larvae, five ganglia per larvae, *P <0.005, Student’s t test). Scale bars, 20 μm. DAPT, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester; DMSO, dimethyl sulfoxide; dpf, days postfertilization GFP, green fluorescent protein. |
|
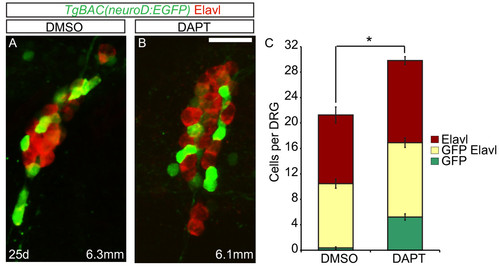
Excess neurons differentiate in late larval dorsal root ganglia (DRG) following Notch inhibition. (A-B) Confocal images of TgBAC(neurod:EGFP) transgenic fish treated with DMSO or DAPT between 20 and 25 dpf. DRG neurons were labeled with anti-GFP antibody (green) and Elavl (red). At 25 dpf, the DRG of a 6.3 mm DMSO-treated fish (A) and a 6.1 mm DAPT-treated fish (B) contain neurons that are labeled with Elavl, GFP/Elavl or GFP antibody alone. (C) Quantification of cells in the DRG. Following DAPT treatment, there is a significant increase in the total number of GFP + and Elavl + cells in the DRG as compared to controls. (n = 10 fish per condition, four ganglia per fish, *P <0.002, Student’s t test). Scale bars, 20 μm. DAPT, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester; DMSO, dimethyl sulfoxide; dpf, days postfertilization GFP, green fluorescent protein. |