- Title
-
GSK-3 Activity Is Critical for the Orientation of the Cortical Microtubules and the Dorsoventral Axis Determination in Zebrafish Embryos
- Authors
- Shao, M., Lin, Y., Liu, Z., Zhang, Y., Wang, L., Liu, C., and Zhang, H.
- Source
- Full text @ PLoS One
|
The dorsalizing activity of lithium treatment during zebrafish early development. (A) A severely dorsalized embryo (radialized). (B) A mildly dorsalized embryo. (C) A normal embryo. (D) Diagram demonstrating the dynamics of the dorsalizing capability of acute lithium treatment (0.3 M LiCl for 8 min). The abscissa axis designates the time at which lithium treatment began. The ordinate axis designates the percentage of three kinds of embryos with different degrees of dorsalization at 12.5 hpf. SW1: Sensitive Window 1; SW2: Sensitive Window 2; UW: Unresponsive Window. The data were obtained in three or more separate experiments, and the number of the embryos used for each data set is more than 100. (E) The dorsalizing effect of the lithium treatment is not caused by osmotic stress by comparing with NaCl treatment at the same salt concentration and treatment time. Embryos in A, B, C and E was at 12.5 hpf, and lateral viewed. The bar in A represents 500 μm. |
|
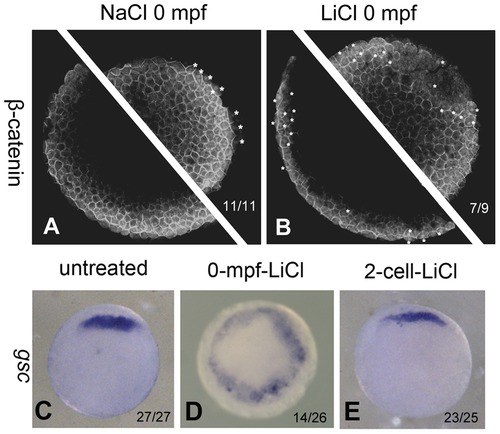
0-mpf lithium treatment activates Wnt/β-catenin signaling at mid-blastula stage and expands the organizer region. (A and B) Confocal immunofluoresence image of of β-catenin in a 0-mpf NaCl treated embryo (A) and a 0-mpf lithium treated embryo (B) To ensure that the entire marginal zone is investigated, each embryo was scanned for two focal planes near the marginal zone of two hemispheres. The embryos were at sphere stage. Nuclear β-catenin was marked with white asterisks. (C–E) Expression pattern of organizer gene gsc in untreated (C), 0-mpf lithium treated (D), and 2-cell-stage lithium treated (E) embryos. All the embryos were animal pole view, dorsal up if it can be distinguished. |
|
0-mpf lithium treatment exacerbates the ventralized phenotype of tokkeabi mutant embryos. (A) Phenotypic analysis of lithium treated tkk embryos. We adopted the Dorsoventral Index previously described [33], but some of the categories were combined in order to simplify the statistics, as stated below: (Aa) V4: a representative radially ventralized embryo; (Ab) V2-V3: a moderately ventralized embryo with distinguishable D–V axis but no eyes; (Ac) C1-Normal-V1: embryos with eyes (regardless of the size) and relatively normal D–V axis; (Ad) C2–C4: A partially dorsalized embryo with shortened anterioposterior length; (Ae) C5: A radially dorsalized embryo. (B–D) the expression of gsc in wild-type (B), tkk mutant (C), and 0-mpf lithium treated tkk mutant embryos (D). (E) The central angle of gsc expression showing a significant decrease in 0-mpf lithium treated embryos with respect to wild-type untreated, 0-mpf lithium treated wild-type and tkk untreated embryos. (F) The measurement of the central angle of gsc expression. The error bars in (E) designate the standard deviation of each data set. ** means that the p value is lower than 0.001 according to the Student′s t test. Embryo numbers were designated for each column in (A) and (E). |
|
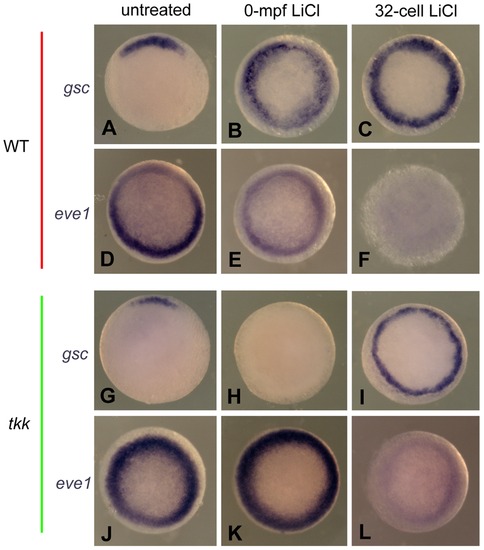
The comparison of dorsal and ventral gene expression between 0-mpf lithium treatment and 32-cell-stage lithium treatment. Representative embryos from indicated groups stained by gsc probe (A–C, and G–I) or eve1 probe (D–F and J–L) at 50% epiboly. All the embryos are animal pole view and with dorsal side upward if it can be distinguished. |
|
0-mpf nocodazole treatment reverses the dorsalizing effect of the 0-mpf lithium treatment. The 0-mpf embryos were treated with 0.35 M LiCl solution in the absence or presence of 0.1 μM nocodazole for 5 min, and then observed at 12 hpf and 22 hpf. 0 35 M NaCl treatment served as control. (A and E) 0-mpf NaCl treated embryos at 12 hpf (A) and 22 hpf (E). (B and F) 0-mpf NaCl and nocodazole co-treated embryos at 12 hpf (B) and 22 hpf (F). (C and G) 0-mpf lithium treated embryos at 12 hpf (C) and 22 hpf (G). (D and H) 0-mpf lithium and nocodazole co-treated embryos at 12 hpf (D) and 22 hpf (H). (I) Statistical data were obtained at 12 hpf for the experiment with embryo numbers on the top of each column. |
|
Paclitaxel treatment on wild-type and tkk mutant embryos. (A) Dorsal view of an untreated 12 hpf embryo. (B) Dorsal view of a 0-mpf paclitaxel treated embryo with ventralized phenotype. (C) An embryo with normal phenotype. (D) An embryo with CE defect like phenotype, showing a smaller head, shorter anterior-posterior axis and malformed somites. (E) A ventralized embryo. Embryos in (C-E) were observed at 24 hpf. The statistical data based on the 24 hpf observation were shown in (F) with embryo numbers shown on the top of each column. |
|
Comparison between lithium and chemical inhibitors of GSK-3 or IMPase in the dorsalizing activity. (A–L) Phenotypic comparison of NaCl, lithium and GSK-3 inhibitor IX exposures. (M–X) Phenotypic comparison of NaCl, lithium and L690, 330 injections. Embryos in A-X were observed at stages indicated at the left side of the figure. (Y) Statistical data of the phenotypic analysis at 11.5 hpf with embryo numbers on the top of each column. (Za–Zf) Examination of gsc expression in 0-mpf NaCl, LiCl, GSK-3 inhibitor IX and L690,330 exposed (Za–Zc) or injected embryos (Zd–Zf). |
|
0-mpf inhibition of GSK-3 activity randomized the parallel microtubule arrays at the vegetal pole and the biased migration of Wnt8a transcripts. Microtubule staining with an anti-β-tubulin antibody to visualize the microtubule arrays formed at around 20 mpf at the vegetal pole. (A) Parallel microtubule arrays detected in NaCl treated embryos. (B) Randomized aligned microtubule arrays detected in 0-mpf lithium treated embryos. (C) Similar phenomenon detected in 0-mpf GSK-3 inhibitor treated embryos. (D–F) 0-mpf lithium and GSK-3 inhibitor IX treatments disrupted the polarized distribution of Wnt8a mRNA observed at 4-cell stage. (D) A 0-mpf NaCl treated wild-type embryo, (E) A 0-mpf lithium treated wild-type embryo, (F) A 0-mpf GSK-3 inhibitor IX treated wild-type embryo (G) Wnt8a mRNA restricted to the animal pole region of the 0-mpf NaCl treated 4-cell-stage tkk mutant embryos. (H I) Exposure of GSK-3 inhibitors failed to alter the distribution of Wnt8a mRNA in 4-cell-stage tkk mutant embryos. |
|
Model of dorsal-ventral axis formation in zebrafish. The zebrafish DV axis specification can be divided to four phases based on the dynamic alteration of the dorsalizing activity of lithium treatment. SW1 designates 0–10 mpf in which lithium treatment can cause dorsalization of the embryos. In this phase, fertilization initiates a GSK-3 dependent mechanism regulating the orientation but not stablization of vegetal microtubules which is critical for the dorsalward transport of DDs like Wnt8a mRNA. UW1 designates the first unresponsive window of lithium treatment, from 10 mpf to the 32-cell stage, in which, especially in the early period, lithium treatment fails to efficiently cause dorsalization. In this period, dorsal determinants are transported from the vegetal pole to the perspective dorsal side. The transduction of Wnt/β-catenin pathway is probably blocked by some unknown mechanism in SW1 and UW1. SW2 designates the period from the 32-cell stage to the mid-blastula stage. In this period, dorsally located DDs are able to inhibit GSK-3, causing the stabilization and nuclear localization of β-catenin, and lead to the expression of dorsal organizer genes. In UW2, lithium treatment loses its ability to dorsalize zebrafish embryos and the organizer gene expression is translated gradually by cell movement to morphologically distinguished dorsal-ventral axis. Arrow head at the lower-left corner indicates the shield. |