- Title
-
Platelet-derived growth factor receptor Beta is critical for zebrafish intersegmental vessel formation
- Authors
- Wiens, K.M., Lee, H.L., Shimada, H., Metcalf, A.E., Chao, M.Y., and Lien, C.L.
- Source
- Full text @ PLoS One
|
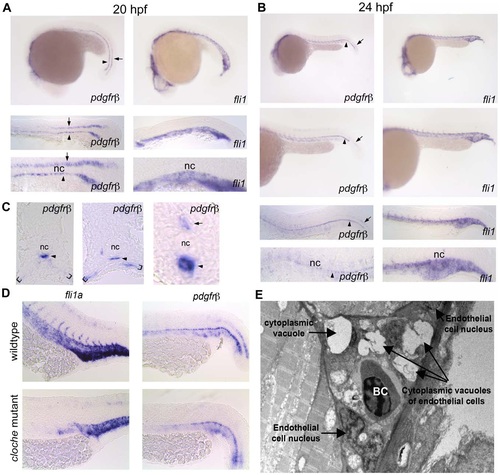
pdgfrβ2 expression in the developing zebrafish. In situ hybridization of embryos at 20 hpf (A) and 24 hpf (B) using an anti-sense probe against fli1a or pdgfrβ2. pdgfrβ2 was expressed in the floorplate (arrow) and the hypochord (arrowhead) at 20 hpf. fli1a is known to be expressed in the tail vasculature. Floorplate expression of pdgfrβ2 diminished by 24 hpf while expression in the hypochord persisted and spread ventrally in association with the dorsal aorta and posterior cardinal vein (B). Cross section analysis further indicated pdgfrβ2 expression in the hypochord (arrowhead), floorplate (arrow) and ventral somite boundary (brackets). cloche mutant embryos showed normal expression of pdgfrβ2 indicating that pdgfrβ2 was not expressed in endothelial cells of the dorsal aorta or posterior cardinal vein (D). Transmission electron micrograph images of a longitudinal section through the ISVs (E). Mural cells surrounding the endothelial cells of the ISVs were absent at 72 hpf. Notochord (nc), blood cell (BC). EXPRESSION / LABELING:
|
|
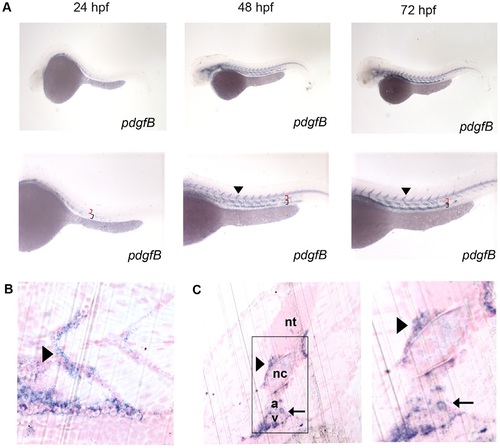
Characterization of pdgf-b in the developing zebrafish embryo. In situ hybridization of embryos at 24, 48, and 72 hpf (A) using an anti-sense probe against pdgf-b. pdgf-b was expressed at all time points in the head and tail vasculature (arrowheads indicate pdgf-b staining in ISVs, red brackets indicate the dorsal aorta, and black brackets indicate the posterior cardinal vein in A). Sagittal (B) and transverse (C) JB-4 sections of 72 hpf embryos analyzed with whole mount in situ hybridization using a probe against pdgf-b. pdgf-b expression was localized near the dorsal aorta, posterior cardinal vein and ISVs (arrow in C indicates pdgf-b staining in dorsal aorta and posterior cardinal vein while the arrowhead indicates staining in the ISV). Neural tube (nt), notochord (nc), dorsal aorta (a), posterior cardinal vein (v). EXPRESSION / LABELING:
|
|
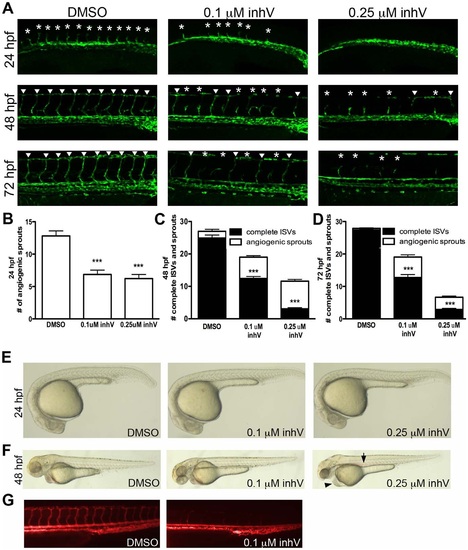
PDGFR inhibition blocked ISV formation and extension. EXPRESSION / LABELING:
|
|
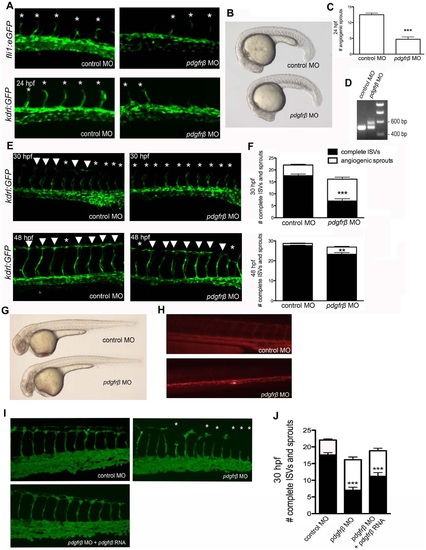
PDGFRβ2 activity was required for ISV angiogenesis in zebrafish embryos. fli1a:eGFP or kdrl:GFP embryos were injected with control morpholino oligonucleotide (MO) or pdgfrβ2 MO at the one-cell stage and imaged at 24 hpf (A), 30 hpf and 48 hpf (E). Light images of whole embryos are shown for 24 hpf (B) and 48 hpf (G). Blocking of splicing was confirmed using RT-PCR (D). pdgfrβ2 MO-injected embryos had significantly more ISV defects than control MO-injected embryos at 24 hpf (A and C), 30 hpf and 48 hpf (E and F). Angiography at 72 hpf showed less circulation through ISVs in pdgfrβ2 MO-injected embryos (H). The ISV defect seen in pdgfrβ2 MO-injected embryos was partially rescued when pdgfrβ2 mRNA was coinjected with pdgfrβ2 MO (I and J). Arrowheads indicate complete ISVs. Asterisks indicate angiogenic sprouts. *** indicates p<0.001, ** indicates p<0.01. All data represent the mean +/- standard error. |
|
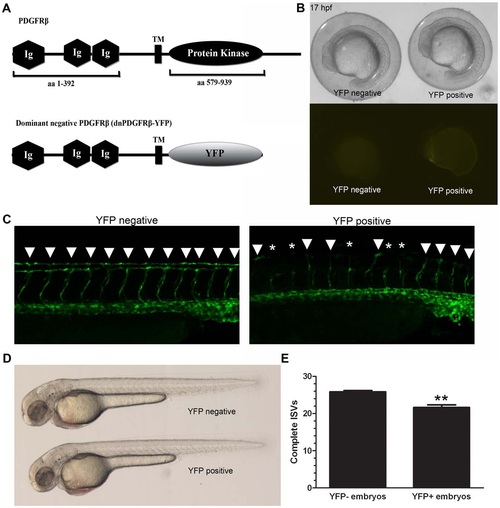
A dominant-negative PDGFRβ blocked ISV angiogenesis in zebrafish embryos. A heat shock-inducible dominant negative form of PDGFRβ was created by substituting the intracellular kinase domains of PDGFRβ with YFP (A, dnPDGFRβ-YFP). Heat shock at 10 hpf led to dnPDGFRβ-YFP expression within 7 hours after heat shock with no effect on overall morphology (B). Heat shock at 20 hpf and analysis at 48 hpf indicated an increase in ISV defects in heat shocked embryos positive for dnPDGFRβ-YFP versus heat shocked embryos negative for dnPDGFRβ-YFP (C and E, n = 23). Embryos heat shocked at 20 hpf that were positive for dnPDGFRβ-YFP at 48 hpf showed no overall morphology defects as compared to embryos heat shocked at 20 hpf negative for dnPDGFRβ-YFP (D). Arrowheads indicate complete ISVs. Asterisks indicate angiogenic sprouts. ** indicates p<0.01. All data represent the mean +/- standard error. EXPRESSION / LABELING:
PHENOTYPE:
|
|
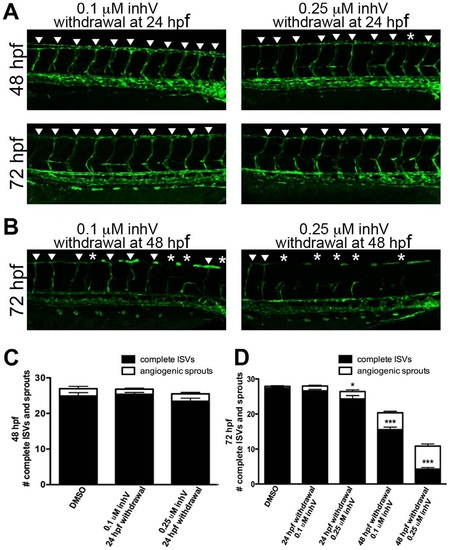
A critical period existed for PDGFR signaling in ISV formation. kdrl:GFP embryos were treated with inhV starting from 6 hpf. Withdrawal of PDGFR inhibition at 24 hpf resulted in full recovery of ISV number and extent within 24 hours after withdrawal (A and C). In contrast, withdrawal of inhV at 48 hpf resulted in little or no recovery of ISV formation by 72 hpf (B and D). Arrowheads indicate fully-formed ISVs. Asterisks indicate angiogenic sprouts. *** indicates p<0.001, * indicates p<0.05. All data represent the mean +/- standard error. |