- Title
-
Inhibitor-resistant type I receptors reveal specific requirements for TGF-beta signaling in vivo
- Authors
- Ho, D.M., Chan, J., Bayliss, P., and Whitman, M.
- Source
- Full text @ Dev. Biol.
|
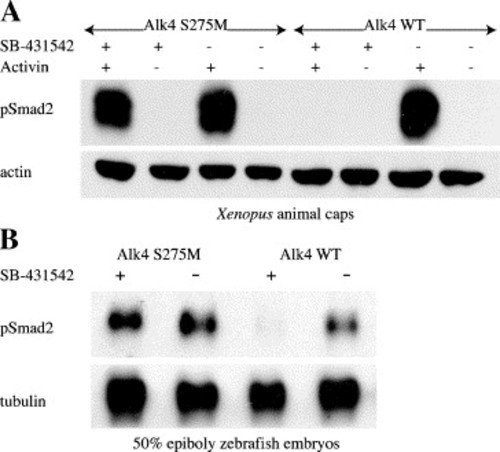
SB-431542 reversibly blocks phospho-Smad2 in Xenopus animal cap explants and zebrafish embryos. Western blots for p-Smad2 were performed on (A) Xenopus animal caps treated with 100 μM SB-431542 or DMSO followed by 0.3 nM soluble activin protein, (B) 50% epiboly zebrafish embryos treated with 100 μM SB-431542 or DMSO at 16-cell stage, and (C) tails from stage 34 embryos treated with 100 μM SB-431542 or DMSO at stage 19 for 24 h and either washed (lane 1) or unwashed (lanes 2 and 3) for 16 h. Cytoskeletal actin was used as a loading control. |
|
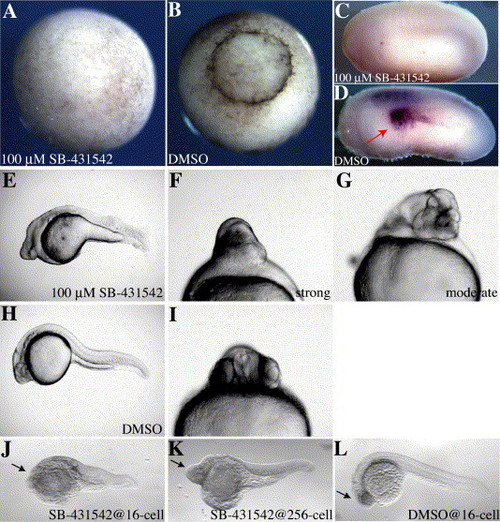
SB-431542 induces morphological defects in embryos. (A–B) Xenopus embryos were treated with SB-431542 (A) or DMSO (B) at stage 3 and allowed to develop until stage 11. Note failure of blastopore lip formation in the treated embryo. (C–D) Xenopus embryos were treated with SB-431542 (C) or DMSO (D) at stage 13 and fixed for in situ hybridization with an xAntivin antisense probe at stage 22. Embryos are photographed with anterior to the left and the left side showing. Note loss of left-sided xAntivin expression (red arrow in panel D) in the treated embryo. (E–I) Zebrafish embryos were treated with 100 μM SB-431542 or DMSO at 16-cell stage and examined at 24 hpf. (E) SB-431542-treated embryo, displaying head and midline defects, fewer and more posterior somites, and poor yolk extension morphology. (F) Close-up of head region of SB-431542-treated embryo with “severe” phenotype. (G) Close-up of head region of SB-431542-treated embryo with “moderate” phenotype. (H) DMSO-treated control embryo. (I) Close-up of head region of control embryo. (J–L) Embryos treated at 16-cell (J) have more severe loss of head and axial structures (arrows) than those treated at 256-cell (K), although both are significantly perturbed compared to controls (L). |
|
SB-431542 treatment alters marker gene expression in zebrafish embryos. Embryos were treated with 100 μM SB-431542 or DMSO at 16-cell stage, and harvested for in situ hybridization with (A, B) goosecoid (gsc), (C, D) no tail (ntl), and (E, F) even-skipped-1 (eve1) at shield stage, (G, H) ntl, (I, J) axial, and (I, J) pax2.1 at bud stage, and (K, L) shh at 18-somite stage. Gsc expression in the dorsal organizer region embryos is severely reduced in treated (A) versus control (B) embryos. In control embryos, ntl expression forms a ring around the margin (D), but expression on the dorsal side is lost in SB-431542-treated embryos (C). In contrast, eve1 expression is not affected (E, F). At bud stage, ntl expression in the tailbud (arrow) is normal, but notochord signal (arrowhead) is absent (G, H). Axial (arrows in panels I and J) expression is also lost in the presumptive notochord / floorplate. Pax2.1 expression is unaffected (arrowheads in panels I and J). By the 18-somite stage, shh expression is still severely reduced, with virtually no signal in the anterior of the embryo (compare panel K to arrow in panel L), and weak, discontinuous staining in the posterior (arrowheads in panel K). Shield stage embryos (A–F) are photographed with the dorsal side to the right. Bud stage embryos (G–J) are photographed with the anterior to the top. EXPRESSION / LABELING:
|
|
Alk4 S275M restores phospho-Smad2 signaling to SB-431542-treated animal caps and embryos. Embryos (Xenopus and zebrafish) were injected with 100 pg Alk4 S275M or 100 pg Alk4 WT. Western blots for phospho-Smad2 were performed on (A) stage 8.5 Xenopus animal caps treated with 100 μM SB-431542 or DMSO followed by 2 nM soluble activin protein, and (B) 50% epiboly zebrafish embryos treated with 100 μM SB-431542 at 16-cell stage. Cytoskeletal actin and tubulin were used as loading controls for panels A and B, respectively. |
|
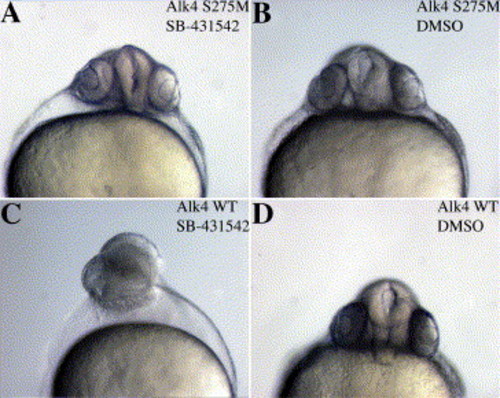
Alk4 S275M rescues the SB-431542-induced phenotype in zebrafish embryos. Zebrafish embryos were injected with (A, B) 100 pg Alk4 S275M or (C, D) 100 pg Alk4 WT, treated with 100 μM SB-431542 or DMSO at 16-cell stage, and photographed at 24 hpf. Alk4 WT embryos display severe anterior defects (C) when treated with SB-431542, but Alk4 S275M embryos appear wild-type (A). DMSO-treated controls (B, C) appear normal, indicating that injection of the receptors alone does not affect morphology. |
|
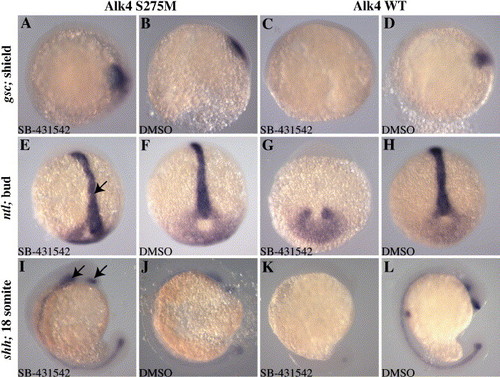
Alk4 S275M rescues marker gene expression in SB-431542-treated embryos. Zebrafish embryos were injected with 50 pg Alk4 S275M or 50 pg Alk4 WT and treated with 100 μM SB-431542 or DMSO at 16-cell stage. Embryos were harvested for in situ hybridization for gsc at shield stage (A–D, dorsal to the right), ntl at bud stage (E–H, anterior to the top), and shh at 18 somite stage (I–L). Alk4 S275M restores gsc expression in SB-431542-treated embryos (A), whereas Alk4 WT did not (C). Notochord expression of ntl at bud stage was restored in Alk4 S275M-injected embryos (arrow in panel E), but not in Alk4 WT embryos (G). Similarly, expression of shh, including anterior domains (arrows in panel I), was rescued in Alk4 S275M embryos (I), but not in Alk4 WT embryos (K). DMSO-treated embryos displayed normal expression patterns for all genes examined (B, D, F, H, J, L). |
|
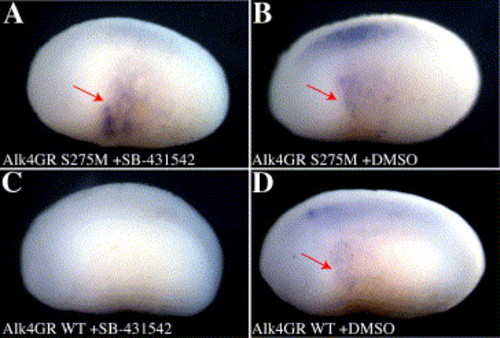
Alk4 S275M rescues left–right patterning in SB-431542-treated Xenopus embryos. Embryos were injected on the left side only with 150 pg xAlk4-GR S275M (A, B) or xAlk4-GR WT (C, D) mRNA and treated with 10 μM Dexamethasone at stage 13.5 followed by 100 μM SB-431542 (A, C) or DMSO (B, D) at stage 14. Embryos were processed for in situ hybridization with an xAntivin antisense probe at stage 22. Embryos were photographed with the anterior to the left and the left side showing. Note specific recovery of antivin expression (red arrow) in the left flank in panel A. |
|
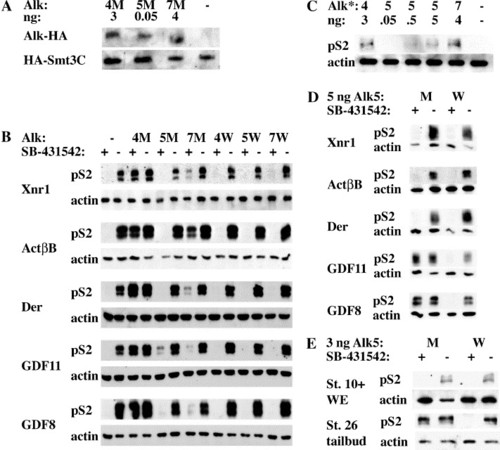
Alk4, Alk5, and Alk7 mediate signaling by different subsets of activin/nodal ligands (A) Embryos were injected with 3 ng Alk4-HA SM (4M), 50 pg Alk5-HA SM (5M), or 4 ng Alk7-SM (7M) along with 500 pg HA-Smt3C as an internal loading control. α-HA IP-Westerns were performed on stage 10.5 embryos. (B) Embryos were injected with 1 ng Xnr1, 20 pg ActβB, 2 ng Derrière, 2 ng cDsl-hGDF11, or 2 ng cDsl-mGDF8 along with 3 ng xAlk4-HA S275M (4M) or WT (4W), 50 pg rAlk5-HA S278M (5M) or WT (5W), or 4 ng rAlk7-HA S270M (7M) or WT (7W). Animal caps were incubated in 100 μM SB-431542 or DMSO until stages 10–10.5, and subsequently harvested for Western blots against p-Smad2. (C) Constitutively active Alks (Alk4*, Alk5*, and Alk7*) were injected at various doses and pSmad2 signaling was assessed in animal caps. (D) 5 ng of rAlk5-HA S278M or rAlk5-HA WT were coinjected with the same concentrations of ligands used in panel A and p-Smad2 signaling was assayed in SB-431542 or DMSO-treated animal caps. (E) Xenopus embryos were injected with 3 ng Alk5-HA S278M or WT. Embryos were treated with 100 μM SB-431542 at stage 6 and harvested at stage 10+, or treated at stage 14 and harvested (tailbud region only) at stage 26. For all experiments, actin was used as a loading control. |
|
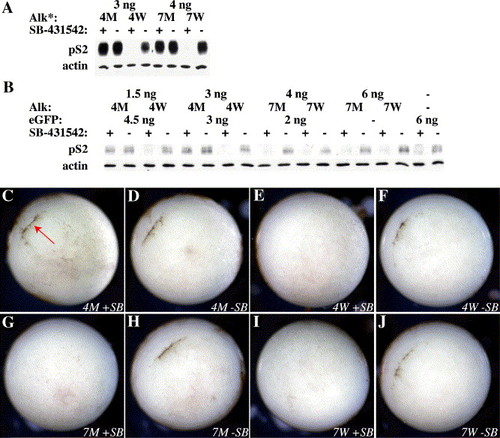
Alk4 but not Alk7 can rescue signaling during gastrulation (A) Alk4* and Alk7*-injected animal caps were treated with 100 μM SB-431542 and p-Smad2 signaling was assessed by Western blot at stage 10.5. (B) Embryos injected with different amounts of xAlk4-HA or rAlk7-HA were treated with 100 μM SB-431542 at stage 6 and harvested for Western blotting at stage 10+. All mRNA levels were equalized to 6 ng total with eGFP. Embryos injected with (C, D) 3 ng xAlk4-HA S275M, (E, F) 3 ng xAlk4-HA WT, (G, H) 4 ng rAlk7-HA S270M, or (I, J) 4 ng rAlk7-HA WT were treated as above and photographed at stage 10+. Note rescue of blastopore lip formation in Alk4 S275M embryo (red arrow in panel B). Embryos are photographed with the vegetal pole showing, dorsal to the upper left. |
Reprinted from Developmental Biology, 295(2), Ho, D.M., Chan, J., Bayliss, P., and Whitman, M., Inhibitor-resistant type I receptors reveal specific requirements for TGF-beta signaling in vivo, 730-742, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.