- Title
-
Epb41l5 competes with Delta as a substrate for Mib1 to coordinate specification and differentiation of neurons
- Authors
- Matsuda, M., Rand, K., Palardy, G., Shimizu, N., Ikeda, H., Nogare, D.D., Itoh, M., Chitnis, A.B.
- Source
- Full text @ Development
|
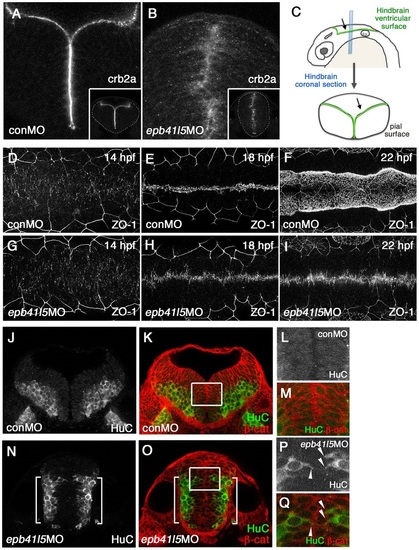
Epb41l5 is required for the formation of the hindbrain ventricle and the proper localization of neurons. (A-C) Coronal sections showing mislocalization of Crb2a in the hindbrain of epb41l5 morphants. Whereas Crb2a is restricted to a well-defined apical/ventricular surface in wild-type embryos injected with control morpholino (MO) (conMO), Crb2a is diffusely expressed in epb41l5 morphants (epb41l5MO). Lower-magnification images are shown in insets. Diagram in C illustrates approximate positions of coronal sections in the hindbrain. (D-I) Failure of organization of ZO-1-positive AJs and formation of the hindbrain ventricle in epb41l5 morphants. Confocal images were taken from the dorsal side of embryos. The anterior is to the left and the posterior is to the right. In control MO embryos, accumulation of ZO-1+ puncta at 14hpf is gradually aligned to the midline of the hindbrain and forms a continuous line at 18hpf. The hindbrain ventricle inflates at 22hpf. In epb41l5 morphants, ZO-1 accumulation at 14hpf is gradually aligned to the midline of the hindbrain but fails to form a continuous line at 18hpf. The hindbrain ventricle is not formed at 22hpf. (J-Q) Coronal sections showing mislocalization of HuC-expressing neurons in epb41l5 morphants. In control MO embryos, HuC-positive neurons are localized at the pial region of the hindbrain and do not have contacts with the ventricular surface. In epb41l5 morphants, a fraction of HuC-positive cells were located near the midline of the hindbrain and maintain contacts with the prospective ‘ventricular’ surface. Boxed areas in K and O are shown at higher magnification in L,M and P,Q, respectively. Brackets indicate HuC-positive neurons which are still localized near the basal/pial surface of the hindbrain. Arrowheads indicate HuC-positive cells which are localized near the midline. |
|
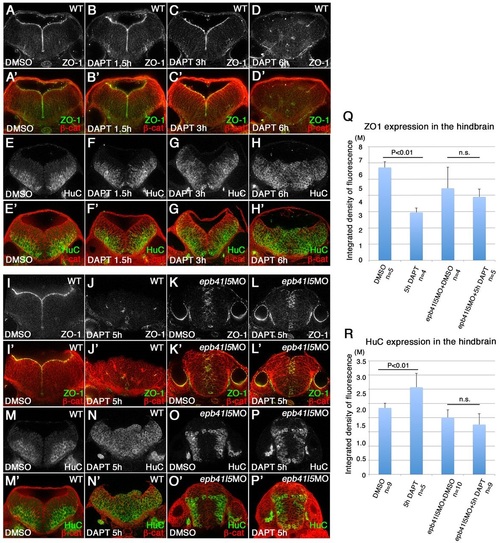
Delays in disassembly of AJs and neuronal differentiation in epb41l5 morphants. (A-H′) Timeline of AJ disassembly (A-D′) and differentiation of neurons (E-H2) during DAPT treatment in wild-type embryos. Embryos were treated with 50µM DAPT for 1.5, 3 or 6h and immunostained at 32hpf. Loss of Notch signaling by DAPT treatment results in loss of AJs and premature differentiation of NPCs into neurons. (I-P′) Delays in AJ disassembly and differentiation of neurons in epb41l5 embryos. Embryos were treated with DAPT for 5h. In control embryos (WT), 5h DAPT treatment eliminates ZO-1 at the apical/ventricular surface, accompanied by a corresponding increase in HuC. In epb41l5 morphants, there is a much smaller reduction in ZO-1 immunostaining and no obvious increase in HuC immunostaining. (Q,R) Quantitative analyses of ZO-1 and HuC expression. Total fluorescence intensities of ZO-1 and HuC in the hindbrain were measured in individual confocal images using ImageJ. Error bars represent s.d. n.s., not significant. M, million integrated pixel intensity. |
|
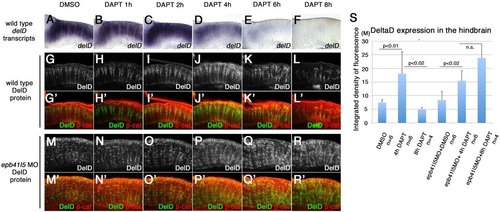
‘Intermediate’ neuronal progenitor population expressing DeltaD is increased in epb41l5-deficient embryos. (A-L′) Timeline of disappearance of DeltaD in progenitors during neuronal differentiation in wild-type embryos. Two hours of DAPT treatment increases expression of deltaD transcripts, followed by increased expression of DeltaD protein at 4h. Expression of deltaD and DeltaD diminishes after 6 and 8h of DAPT treatment. (M-R′) Continued expression of DeltaD in epb41l5 morphants. Increased DeltaD expression persists after 8h of DAPT treatment. (S) Quantitative analysis of DeltaD expression. Total fluorescence intensity of DeltaD in the hindbrain was measured in individual confocal slices using ImageJ. Error bars represent s.d. n.s., not significant. M, million integrated pixel intensity. |
|
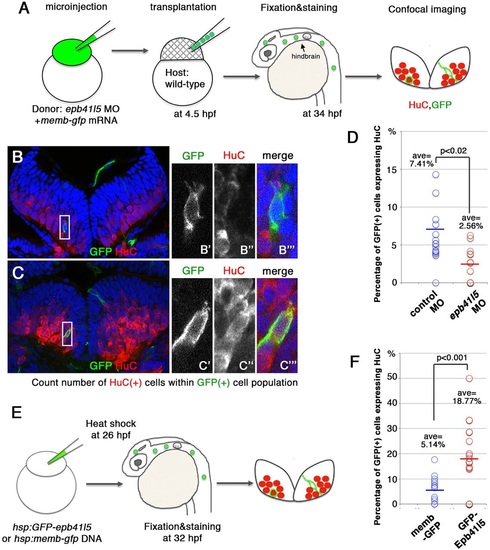
Epb41l5 facilitates differentiation of neurons. (A) Schematic of the experiment. epb41l5 morpholino was injected into one-cell-stage embryos with memb-gfp mRNA. Twenty to thirty cells were transplanted to wild-type embryos. Ratios of HuC-expressing cells within total GFP-expressing transplanted cells were analyzed in individual confocal images. (B,C) Representative images of HuC-positive neurons in the hindbrain. Boxed areas in B and C are enlarged in the right-hand panels. The cell in B does not express HuC, suggesting an undifferentiated NPC. The cell in C does express HuC, suggesting a differentiated neuron. (D) Reduced expression of epb41l5 attenuates differentiation of neurons. Out of the 428 control transplanted cells in 16 embryos that were analyzed, 7.41% cells expressed HuC. Out of the 221 epb41l5-deficient transplanted cells in 12 embryos that were analyzed, 2.56% cells expressed HuC. ave, average. (E) Schematic of the experiment. Plasmid DNAs encoding membGFP or GFP-Epb41l5 were microinjected. At 6h after heat-shock treatment, HuC expression in GFP-expressing cells was analyzed at 32hpf. (F) GFP-Epb41l5 facilitated differentiation of neurons. Out of the 623 membGFP-expressing cells in eight embryos that were analyzed, 5.14% cells expressed HuC. Out of the 277 cells overexpressing GFP-Epb41l5 in six embryos that were analyzed, 18.77% cells expressed HuC. |
|
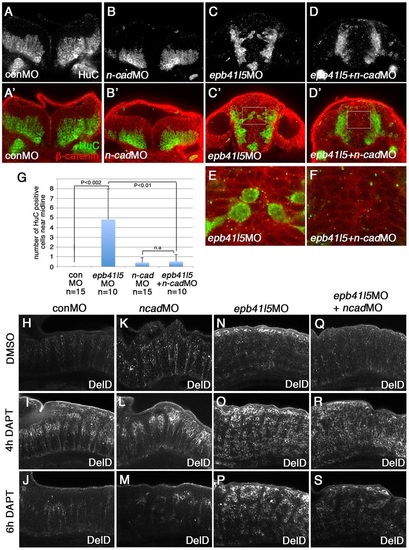
Reduced expression of n-cadherin rescues a delay of delamination and differentiation of neurons in epb41l5-deficient embryos. (A-F) Coronal sections showing localization of HuC-expressing neurons in the hindbrain. HuC-positive cells are only localized at the pial side of the hindbrain in control MO- or 0.1ng n-cadherin MO-injected embryos. A fraction of HuC-positive cells are mislocalized near the midline in epb41l5 morphants. The mislocalization of HuC-positive cells is rescued by partial knockdown of n-cadherin. The boxed areas in C′ and D′ are enlarged in E and F, respectively. (G) Statistical analysis of distribution of HuC-positive cells in the hindbrain. Error bars represent s.d. n.s., not significant. (H-S) DeltaD expression in the hindbrain. Lateral views. After 4h of DAPT treatment DeltaD expression is increased. After 8h of DAPT treatment, whereas DeltaD expression is decreased in control embryos and n-cadherin morphants, DeltaD expression persists in epb41l5 morphants. The persistent expression of DeltaD in epb41l5 morphants is rescued by partial knockdown of n-cadherin. |
|
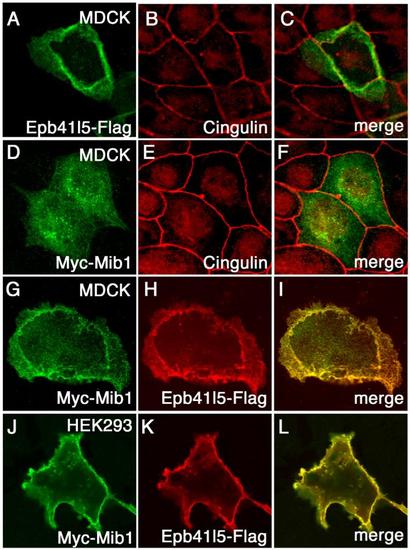
Epb41l5 changes subcellular distribution of Mib1 (A-F) Epb41l5 is a membrane-associated protein and Mib1 is a cytoplasmic protein. Flag-Epb41l5 or Myc-Mib1 was transfected in MDCK cells and their cellular distribution was examined. Cell-to cell junction is labeled by Tight Junction protein, Cingulin. (G-I) Epb41l5 changes cellular distribution of Mib1 from the cytoplasm to the basolateral membrane in MDCK cells. (J-L) Epb41l5 changes cellular distribution of Mib1 from the cytoplasm to the plasma membrane in HEK293 cells. |
|
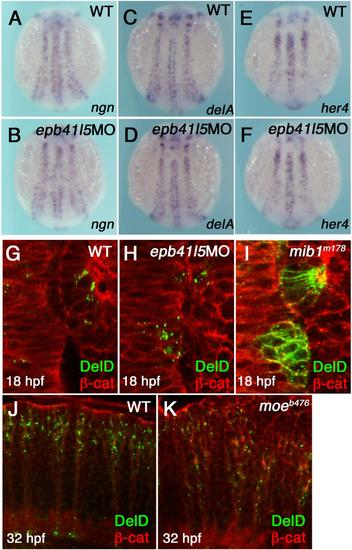
Epb41l5 does not modify Notch signaling or subcellular distribution of DeltaD in the neuroepithelium. (A-F) Reduced expression of epb41l5 does not change fate specification of neurons mediated by Notch-mediated lateral inhibition. ngn: bHLH proneural transcription factor, delta: Notch ligand, her4: direct outcome of Notch signaling. 3-somite stage embryos. (G-I) Reduced expression of Epb41l5 does not change subcellular distribution of DeltaD in the hindbrain neuroepithelium. Dorsal views of the 4th rhombomere and the otic placode in 10-somite stage embryos. DeltaD is mainly localized in cytoplasmic puncta in wild-type embryos. DeltaD is mainly localized on the plasma membrane in mib1m178 mutants, confirming that Mib1 facilitates endocytosis of DeltaD in neuroepithelial cells. DeltaD is mainly localized in cytoplasmic puncta in epb41l5 deficient embryos, suggesting that Epb41l5 is not essential for Mib1-mediated endocytosis of Delta. (J,K) Loss of Epb41l5 does not change subcellular distribution of DeltaD in the hindbrain in epb41l5 mutant moeb476. Lateral view of the 4th rhombomere at 32 hpf. |
|
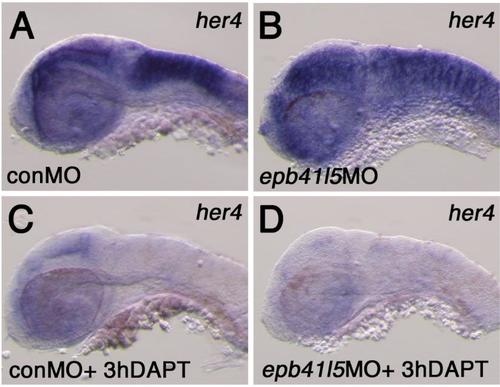
Expression of her4 in the hindbrain of epb41l5 deficient embryos (A,B) Reduced expression of epb41l5 does not significantly change expression levels of her4 in epb41l5 morphants. Lateral view of the hindbrain at 32hpf. (C,D) DAPT treatment effectively reduces her4 expression in epb41l5 morphants as in control embryos. Lateral view of the hindbrain at 32hpf. |
|
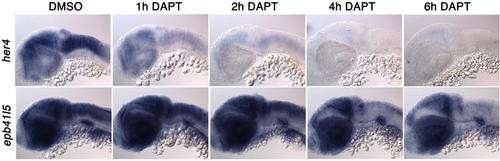
Notch signaling changes the numbers of cells expressing ebp41l5 DAPT reduces expression of ebp41l5 in the hindbrain. Embryos were treated with DAPT for 1, 2, 4 and 6 hours. Lateral view of the hindbrain. 32 hpf |
|
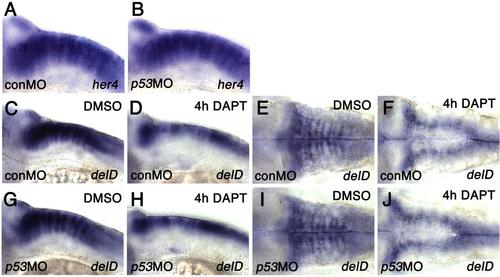
Notch signaling changes the numbers of cells expressing ebp41l5 (A-B) p53 knockdown does not change expression of her4, a direct target of Notch signaling at 32 hpf. (C-J) p53 knockdown does not change expression of deltaD in DAPT-treated embryos. Lateral views of the hindbrain (C,D,G,H) and dorsal views of the hindbrain (E,F,I,J) at 32 hpf. After 4 hours of DAPT treatment, deltaD expression in the hindbrain paraboundary domains is downregulated and the expression is limited to the hindbrain rhombic lip, suggested that DAPT effectively inhibits Notch signaling and facilitates differentiation of DeltaD-positive NPCs determined to become neurons in p53 deficient embryos. |