- Title
-
Innervation is required for sense organ development in the lateral line system of adult zebrafish
- Authors
- Wada, H., Dambly-Chaudière, C., Kawakami, K., and Ghysen, A.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
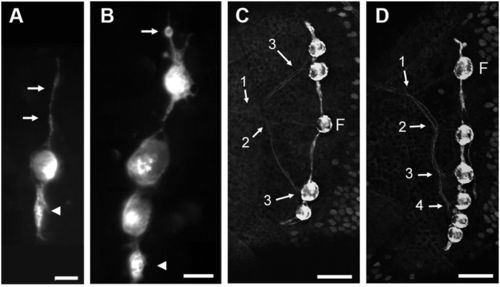
Stitches in zebrafish. (A and B) Initiation of stitch formation in cxcr4.139:rfp fish. Long cell processes extend from the founder neuromast along the dorsoventral axis (A, arrows) and progressively swell up (arrowhead). Cell proliferation (B, arrow: cell rounding up before mitosis) eventually leads to the formation of a new, complete accessory neuromasts (B, arrowhead). (C and D) Innervation of stitches in Hgn39d; ET20 fish. The branching pattern reveals the history of stitch formation. Numbers refer to the successive branching/budding events. F: founder neuromast. In all figures, anterior is left and dorsal is up. (Scale bars: A and B—20 μm; C and D—50 μm.) |
|
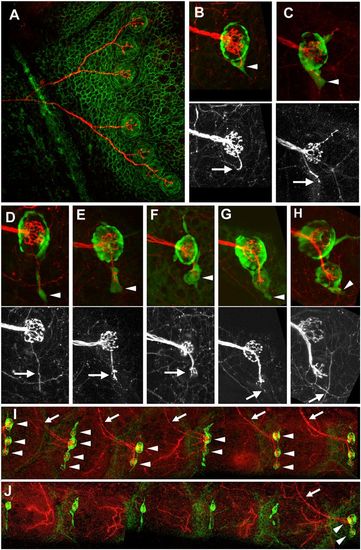
Innervation and budding. (A) Single neuron labeled through plasmid injection in a cldnb:gfp background. (B–H) Successive stages in the formation of the first bud neuromast as seen in nbt-dsred, ET20 fish. Arrowheads: cellular extensions; arrows: accompanying neurite. (I and J) Effect of denervation on stitching. (I) Control side. (J) denervated side. Arrowheads: neuromasts; arrows: neurites. The remaining neurites in J belong to the cutaneous sensory system and are unrelated to the lateral line. (Scale bars: A, 50 μm; B–G, 20 μm; H and I, 100 μm.) EXPRESSION / LABELING:
|
|
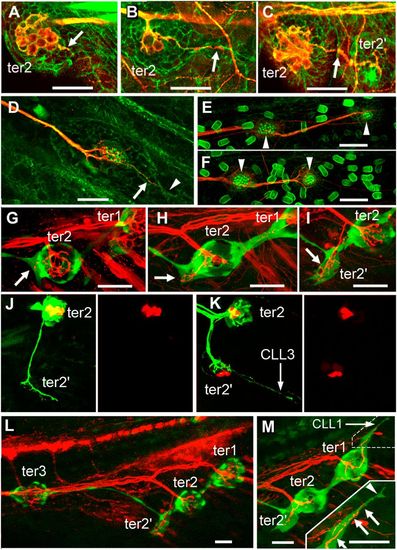
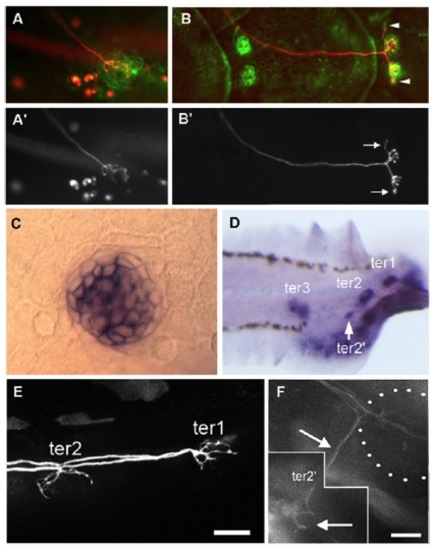
Development of the terminal and caudal systems. (A–F) terminal and caudal budding in Thunnus simultaneously labeled for actin by phalloidin labeling and for acetylated tubulin by immunolabeling. (A and B) Neurite extension from ter2 (arrow) prefigures the formation of bud-neuromast ter22 in Thunnus. (C) ter2′ has formed and remains neurally connected to ter2 (arrow). (D) First neuromast of a caudal line with a neurite branch (arrow) extending into a budding extension (arrowhead). (E and F) First two neuromasts (arrowheads) of the two dorsalmost CLL lines of the same larva, showing that the caudal lines form by sequential budding. (G–M) Terminal and caudal budding in Danio. (G–I) Ventral migration of ter2 and extension of a neurite in the direction where ter2′ will form (arrows), as visualized in 12-dpf nbt-dsred; ET20 fish. (J and K) Formation of hair cell precursors (red) takes place only in the budding structure at 12–13 dpf, after the neurite (green) has sent a branch to presumptive tail line CLL3, as visualized in Hgn39d; atoh-tomato fish. (L) Overall pattern of ter neuromasts when bud-neuromast ter2′ appears, as visualized in nbt-dsred; ET20 fish. (M) Onset of the budding of tail line CLL1 from ter1. (Inset) Higher magnification of the dash-outlined area. A neurite (arrows) extends along the cell process (arrowhead) that emanates from ter1 to initiate CLL1. (Scale bars in A–M: 20 μm.) |
|
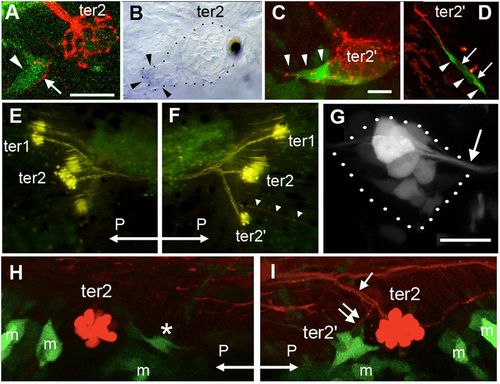
Reporting Wnt and Rspo2 activity during budding. (A) Wnt activity (green) prefiguring ter2′, next to ter2, in a 9-dpf larva. Wnt activity (arrowhead) is adjacent to extending neurites revealed by photoconverted HuC-Kaede (arrow). (B) Presence of lef1 mRNA in the ter2′ budding process. (C) Wnt activity (green) in cells emanating from ter2′ and prefiguring the first ventral line of the caudal system in 10-dpf larvae (arrowheads). (D) Budding of the first ventral line of the caudal system. Wnt activity is detected in budding cells (arrowheads) but not in ter2′ anymore and is tightly correlated to neurite extension (arrows). (E) Absence of ter2′ after overexpression of the Wnt antagonist Dkk. On the contralateral side (F), ter2′ formed normally and the CLL3 line also developed (arrowheads). Hair cells and sensory axons are revealed by DiAsp labeling. (G) Rspo2 enhancer trap activity in the PLL ganglion (outlined in dotted lines). Arrow: root of the PLL nerve extending to the neuromasts. (H) After nerve cut, Wnt activity is severely reduced next to ter2 (asterisk), compared with the control side (I). Arrow: branch of the PLL nerve innervating ter2; double arrow: branchlet innervating ter2′. “m” indicates GFP-expressing mesenchymal cells that contribute to fin development. Exceptionally, posterior (“P”) is left in E and H. |
|
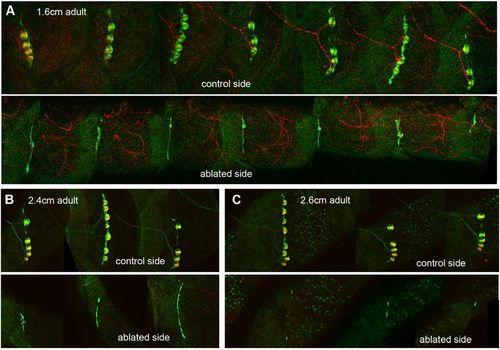
Degeneration of neuromasts in the absence of innervation. (A) Control and experimental sides of a 2-mpf nbt-dsred, ET20 fish; the PLL ganglion had been ablated at 5–7 dpf in the experimental side. (B and C) Control and experimental sides of Hgn39d, ET20, DiAsp-labeled fish where the PLL ganglion had been ablated at 5–7 dpf. (B) Three-month post fertilization, 2.4-cm fish. (C) Three-month post fertilization, 2.6-cm fish. (Scale bars: 500 μm.) |
|
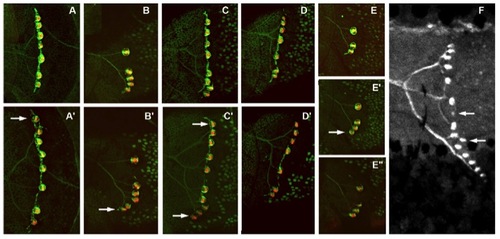
Growth of stitches can occur through addition of a dorsal bud neuromast (A and A′) or a ventral one (B and B′) or both (C and C′). Long periods can go by without any increase in stitch size (1 mo between D and D′). Conversely, a bud neuromast may be added in 2 wk, without anymore budding over the next 2 mo (E–E′ ′). Bud neuromasts may also be formed by other bud neuromasts within a stitch (F). (A and A′) Same fish at 3 mpf, 25 mm and at 4 mpf, 28 mm. (B–D) Same fish at 3 mpf, 25 mm and at 4 mpf, 29 mm. (B and B′) Stitch on somite 17. (C and C′) Stitch on somite 18. (D and D′) Stitch on somite 20. (E) Three-months post-fertilization, 26-mm fish. (E′) Ten days later, same size. (E′ ′) One month later, 28 mm. (F) Five-months post-fertilization fish, 3.1 cm. Arrows point to new bud neuromasts. (A–E) Maximal projections from confocal stacks. (F) Fluorescence image. |
|
(A and B) Single-neuron labeling through plasmid injection in a cldnb-gfp embryo. (A and A′) At 5 days post fertilization (dpf), the neuron innervates a single neuromast. (B and B′) Same fish 1 mo later. The founder neuromast is flanked by a fully differentiated bud neuromast, and both neuromasts are generating a new bud on either side. In both cases, neurites (arrows) invade the bud structures (arrowheads) well before the bud neuromasts begin to differentiate. (C and D) Expression of lgr6 in neuromast cells at 3 dpf (C) and at 11 dpf (D). [Scale bars: 20 μm (C) and 10 μm (D).] (E and F) Rspo2 enhancer trap activity in the neurites that innervate ter1 and ter2 at 7 dpf (E) and in a branch innervating bud neuromast ter2′ at 11 dpf (F). (F) Composite of two frames from a confocal stack, 5.2 μm apart. Maximum projection cannot be used because of high reporter activity in the surrounding mesenchyme. EXPRESSION / LABELING:
|
|
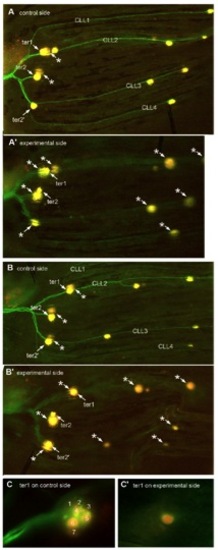
Caudal fin lines do not form in the absence of innervation as visualized in Hgn39d fishes where the hair cells are labeled with DiAsp; asterisks mark out-of-focus neuromasts on the contralateral side. (A) ter2′ and all four caudal lateral lines (CLL) lines are present on the control side of 40-dpf fish, but not after complete ganglion ablation at 5 dpf on the experimental side (A′). (B) Even when ter2′ is present, due to ganglion ablation being completed only at 10 dpf, all CLL lines are still absent on the experimental side (B′). (C) A case of extreme reduction in the number of labeled hair cells in a denervated ter1 neuromast. At least seven hair cells were labeled on the control side (C), and only one was seen on the experimental side (C′). In A and B, ablation was performed on the left ganglion; both sides were photographed independently, and the figure showing the control side was subsequently inverted to facilitate comparison with the experimental side. A and B are assembled from consecutive frames of Z-stacks; C shows single frames. |
|
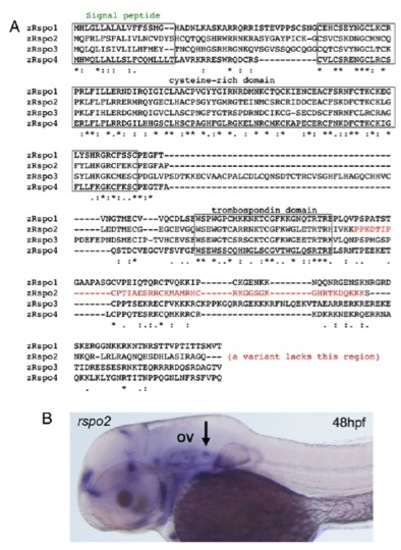
Sequence of the R-spondin (Rspo) gene family (A) and expression of rspo2 (B). The arrow points to the posterior lateral line ganglion, posterior to the otic vesicle (“OV”). EXPRESSION / LABELING:
|