- Title
-
Parkin Is Protective against Proteotoxic Stress in a Transgenic Zebrafish Model
- Authors
- Fett, M.E., Pilsl, A., Paquet, D., van Bebber, F., Haass, C., Tatzelt, J., Schmid, B., and Winklhofer, K.F.
- Source
- Full text @ PLoS One
|
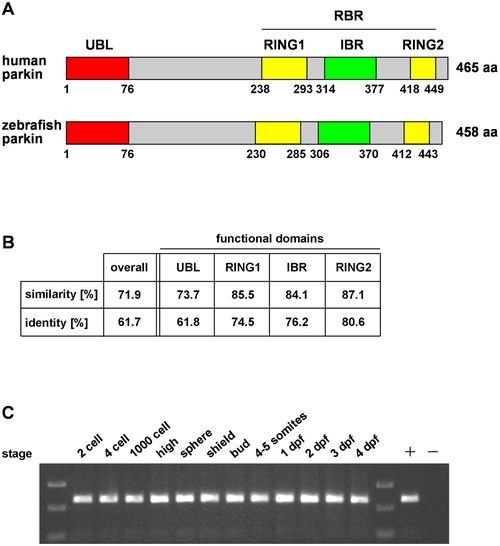
The zebrafish parkin orthologue is highly conserved and expressed during development. (A) Modular structure of human and zebrafish parkin. UBL: ubiquitin-like domain, RING: really interesting new gene, IBR: in-between RING, RBR: RING between RING fingers. (B) Protein sequence alignment of zebrafish parkin and human parkin was analyzed in regard to similarity and identity using BOXSHADE 3.21. Analysis of the functional domains of parkin reveals a higher degree of identity/similarity compared to the overall sequence. (C) Parkin mRNA expression in developing zebrafish. Total mRNA was reversely transcribed into cDNA and analyzed by PCR using parkin-specific primers; + positive control: zebrafish parkin plasmid DNA as a template; - negative control without template. dpf: days post fertilization. |
|
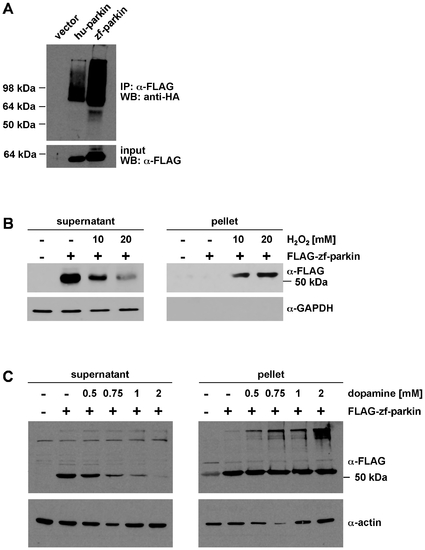
Zebrafish parkin promotes auto-/transubiquitylation and is prone to misfolding in the presence of high level oxidative stress. (A) Zebrafish parkin shows auto-/transubiquitylation activity. HEK293T cells were co-transfected with HA-ubiquitin and either FLAG-tagged human parkin (hu-parkin) or FLAG-tagged zebrafish parkin (zf-parkin). The cells were treated with the proteasomal inhibitor MG-132 overnight. At 24 h after transfection, cells were harvested, lysed under denaturing conditions, and cleared by centrifugation. The supernatants were subjected to an immunoprecipitation (IP) using anti-FLAG agarose beads. The immunoprecipitates were resolved by SDS-PAGE and immunoblotted with an anti-HA mAB (WB). Aliquots of the supernatants were removed before immunoprecipitation and were immunoblotted with anti-FLAG mAB (input, lower panel). (B) SH-SY5Y cells were transiently transfected with FLAG-tagged zebrafish parkin (FLAG-zf-parkin) and exposed to oxidative stress (10 and 20 mM H2O2, 30 min). The cells were then lysed in detergent buffer (0.1% Triton X-100 in PBS), and fractionated into detergent-soluble (supernatant) and -insoluble (pellet) fractions by centrifugation. Parkin present in the supernatant and pellet fraction was analyzed by Western blotting using an anti-FLAG monoclonal antibody. GAPDH (only present in the soluble fraction) was used as a loading control. (C) SH-SY5Y cells were transiently transfected with FLAG-tagged zebrafish parkin (FLAG-zf-parkin) and exposed to dopamine stress (0.5, 0.75, 1 and 2 mM, 5 h). The cells were then lysed in detergent buffer (0.1% Triton X-100 in PBS), and detergent-soluble and -insoluble fractions were analyzed as described in (B). Actin was used as a loading control. |
|
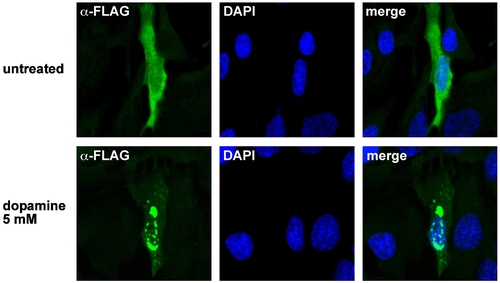
Dopamine induces aggregation of zebrafish parkin. SH-SY5Y cells grown on glass coverslips were transiently transfected with FLAG-tagged zebrafish parkin. 24 h after tansfection, the cells were exposed to dopamine (5 mM, 5 h). The intracellular distribution of zebrafish parkin was analyzed by indirect immunofluorescence of permeabilized cells using an anti-FLAG monoclonal antibody (green). Nuclei were stained by DAPI (blue). |
|
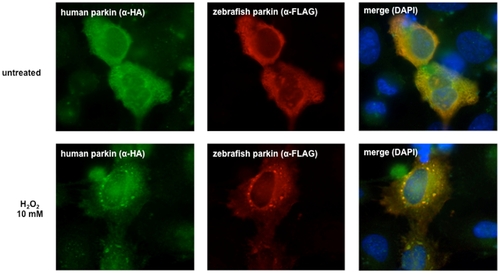
Human and zebrafish parkin co-aggregate. HeLa cells grown on glass coverslips were transiently transfected with FLAG-tagged zebrafish parkin and FLAG-tagged human parkin. 24 h after transfection the cells were exposed to hydrogen peroxide (10 mM) for 30 min. The cells were then fixed and analyzed by indirect immunofluorescence using an anti-HA polyclonal and an anti-FLAG monoclonal antibody. Nuclei were stained by DAPI (blue). |
|
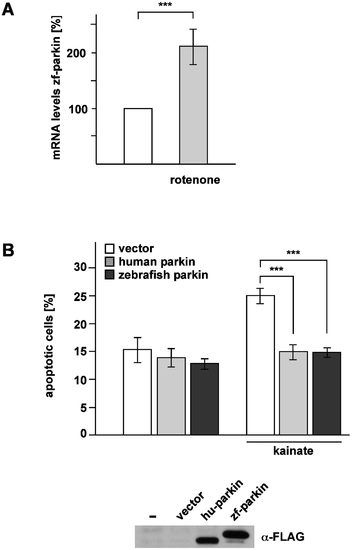
Zebrafish parkin is stress-inducible and stress-protective. (A) Zebrafish parkin is transcriptionally up-regulated in response to mitochondrial stress. Pac2 cells (zebrafish fibroblasts) were incubated with rotenone (10 nM) for 1 h and harvested 6 h after treatment. Total cellular RNA was isolated and subjected to semi-quantitative real-time PCR using zebrafish parkin-specific primers. The amount of RNA of each sample was normalized with respect to the endogenous housekeeping control gene β-actin. Shown is the fold increase in the amount of zebrafish parkin-specific mRNA compared with the untreated control based on 5 independent experiments. (B) Zebrafish parkin protects cells from excitotoxin-induced cell death. SH-SY5Y cells were co-transfected with EYFP and either vector, FLAG-tagged human parkin (hu-parkin) or FLAG-tagged zebrafish parkin (zf-parkin). 24 h after transfection, the cells were incubated with kainate (1 mM) for 3 h, fixed, permeabilized, and analyzed by indirect immunofluorescence using an antibody against active caspase-3. Shown is the percentage of apoptotic cells among at least 300 transfected cells. The quantification is based on three independent experiments performed in duplicates. As a control for parkin expression, aliquots of the cell lysates were immunoblotted with an anti-FLAG monoclonal antibody (lower panel). ***p<0.001 |
|
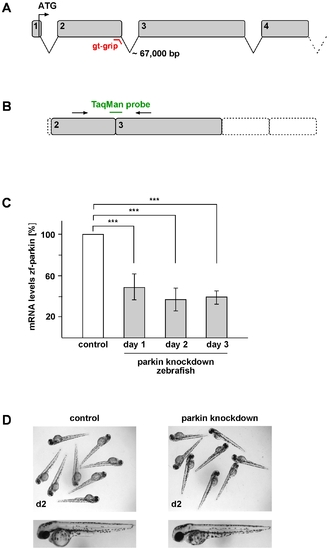
Transient knockdown of parkin in zebrafish does not lead to gross morphological alterations. (A) Genomic structure of zebrafish parkin comprising a total of 12 exons. The splice-blocking GT-grip targets the exon-intron junction of exon 2. (B+C) Verification of the parkin knockdown efficiency by semi-quantitative real-time PCR. (B) The TaqMan™ probe targets the exon 2/exon 3 junction and cannot bind in case of incorrect splicing. (C) Parkin knockdown efficiency at day 1, 2 and 3 after injection of the parkin-specific GT-grip in comparison to a control GT-grip. The amount of zebrafish parkin-specific mRNA was normalized to β-actin in parkin GT-grip-injected zebrafish compared to control GT-grip-injected zebrafish. Quantification is based on 4 independent experiments. ***p<0.001 (D) Parkin-deficient zebrafish embryos do not show obvious morphological alterations. Two-day-old parkin knockdown zebrafish embryos are shown in comparison to control-injected embryos. |
|
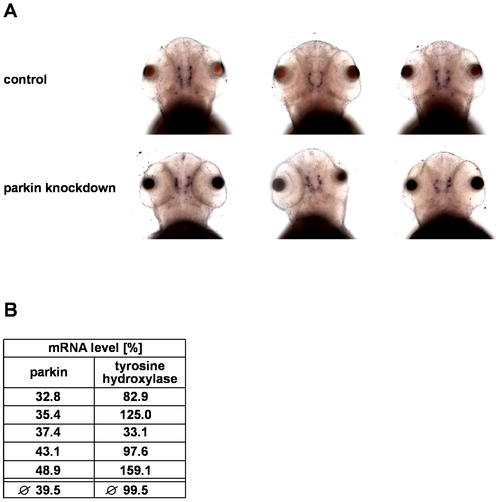
Parkin deficiency has no effect on tyrosine hydroxylase levels in zebrafish. (A) In situ hybridization using an antisense RNA probe for tyrosine hydroxylase (TH) as a marker for dopaminergic neurons. Staining of parkin knockdown and control-injected zebrafish larvae shows large interindividual variations in both the control and parkin knockdown group. Dorsal views of three-day-old larvae. (B) There is no correlation between the efficiency of parkin knockdown and TH mRNA levels. TH mRNA levels were quantified in parkin-knockdown zebrafish using semi-quantitative real-time PCR. The amount of zebrafish TH-specific mRNA was normalized to β-actin in parkin GT-grip-injected zebrafish larvae compared to control-injected embryos at day 3 after injection.The graph shows the results of 5 independent experiments. In each experiment 10–15 zebrafish larvae per group were analyzed. EXPRESSION / LABELING:
|
|
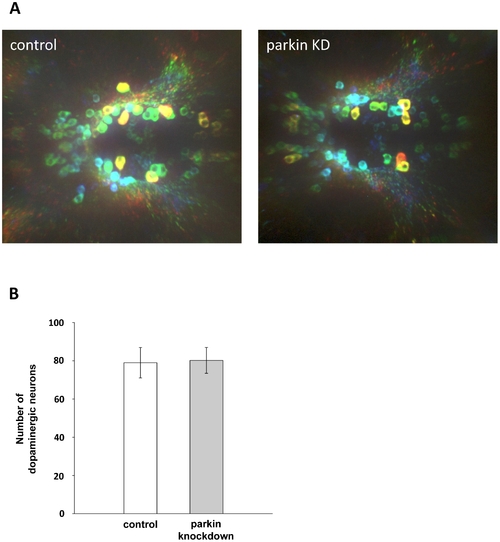
Parkin deficiency does not induce a loss of diencephalic dopaminergic neurons. (A) Whole mount immunostaining using an antibody for tyrosine hydroxylase (TH) as a marker for dopaminergic neurons. Staining of control GT-grip-injected and parkin GT-grip-injected zebrafish larvae shows no difference in the number of dopaminergic neurons. The neurons are depth-color-coded to illustrate the vertical position of the individual neurons in the brain and to allow the discrimination of neurons located on top of each other. Red neurons are located most dorsally, followed by yellow, green, blue and violet. Dorsal views of three-day-old larvae, anterior to the left. (B) Quantification of TH-positive dopaminergic neurons from 27 control and 27 parkin knockdown zebrafish larvae (parkin knockdown efficiency: 55%). TH-positive neurons were counted in a blinded manner, i.e. the researcher was blind to the knockdown status of the zebrafish larvae. EXPRESSION / LABELING:
|
|
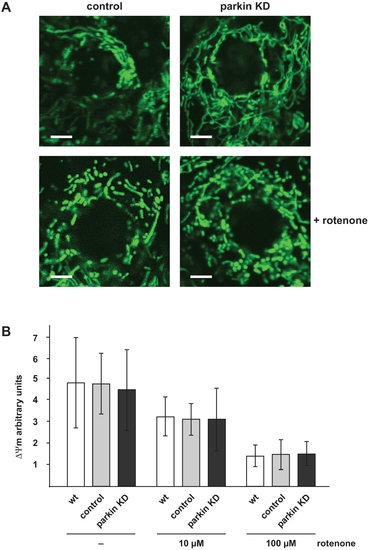
Mitochondrial morphology and membrane potential is not affected in parkin-deficient zebrafish. (A) Mitochondrial morphology in not altered in parkin knockdown zebrafish embryos. Live cell imaging of mitochondria in one-day-old parkin-deficient zebrafish and control-injected zebrafish. Mitochondria were visualized by the transient expression of GFP targeted to mitochondria (green). A transient knockdown of parkin in zebrafish did not cause mitochondrial fragmentation in the outer skin of zebrafish embryos (upper images). Mitochondrial fragmentation induced by rotenone (100 μg/l, 6 h) was not aggravated in parkin-deficient zebrafish embryos (lower images). Scale bar 5 μm. (B) The membrane potential of total mitochondria isolated from parkin-deficient zebrafish embryos is not altered in comparison to mitochondria isolated from wildtype or control-injected embryos, neither under basal conditions nor after rotenone treatment. Isolated mitochondria from one-day-old wildtype, control-injected and parkin knockdown zebrafish were either left untreated or stressed with different concentrations of rotenone and subsequently incubated with the fluorescent dye JC-1. The mitochondrial membrane potential is represented as the ratio of intra-mitochondrial JC-1 (orange) and cytosolic JC-1 (green). Quantification is based on three independent experiments. KD: knockdown. |
|
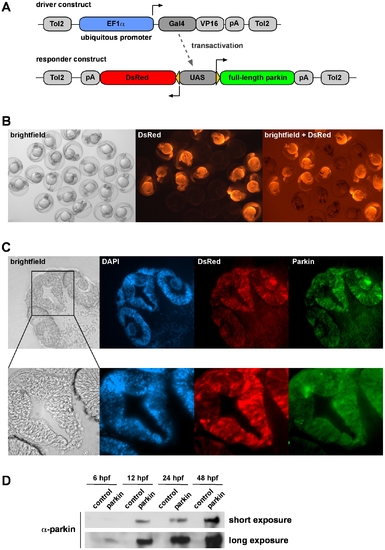
Generation of transgenic parkin zebrafish. (A) A Gal4/UAS-based bidirectional expression system consisting of two constructs was used for the generation of transgenic parkin zebrafish. The driver construct contains the ubiquitous promoter EF1α, driving the expression of Gal4/VP16, which binds to the upstream activating sequence (UAS) of the responder construct. Activation of the UAS leads to the expression of both full-length parkin and DsRed. (B) DsRed fluorescence in living offspring from transgenic zebrafish outcrossed with wildtype zebrafish. One-day-old transgenic zebrafish characterized by ubiquitous expression of DsRed can be distinguished from their non-transgenic siblings. (C) Parkin and DsRed show an overlapping expression pattern in transgenic zebrafish. Cryosections of the head of an one-day-old transgenic parkin zebrafish were analyzed for parkin expression (immunostaining with the anti-parkin mAb PRK8) and DsRed fluorescence. Cell nuclei were stained by DAPI. (D) Western blot analysis of transgenic parkin zebrafish and their non-transgenic siblings (control). Embryos were lysed at different time points and parkin present in the lysates was detected by immunoblotting using the anti-parkin mAb PRK8. hpf: hours post fertilization. EXPRESSION / LABELING:
|
|
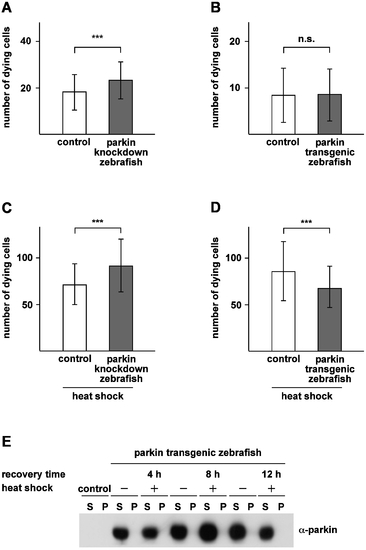
Parkin protects zebrafish from heat shock-induced cell death. (A) Basal cell death is slightly increased in parkin-deficient zebrafish. Two-day-old parkin knockdown zebrafish embryos and control-injected embryos were analyzed for dying cells in the spinal cord (neuronal and non-neuronal) by staining with acridine orange. Parkin-deficient zebrafish embryos showed a small but significant increase in basal cell death in comparison to control-injected embryos (23.20±7.96 versus 18.19±7.65 dying cells). (B) There is no difference in the rate of cell death between parkin transgenic zebrafish and non-transgenic littermates (8.37±5.82 versus 8.56±5.59 dying cells). (C+D) Parkin-deficient zebrafish are more vulnerable to thermal stress, while transgenic parkin zebrafish are more resistant to thermal stress. 48 hours post fertilization zebrafish embryos were exposed to a heat shock (39°C) for 1 h followed by an 8 h recovery period at 29°C. Staining with acridine orange was used to quantify dying cells (neuronal and non-neuronal) in the spinal cord. Cell death in response to thermal stress was increased in parkin knockdown zebrafish compared to control-injected fish (91.47±28,32 versus 71.0±21.96 dying cells) (C) and decreased in transgenic parkin zebrafish versus non-transgenic siblings (85.51±31.81 versus 67.39±22.26 dying cells) (D). Quantification was based on 3 independent experiments. For each experiment at least 50 embryos per group were analyzed. ***p<0.001. (E) Thermal stress in zebrafish had no effect on the detergent solubility of parkin. Western blot analysis of two-day-old transgenic parkin zebrafish embryos which were either heat shocked with subsequent recovery periods of 4 h, 8 h and 12 h, respectively, or remained untreated. After lysis of zebrafish embryos in detergent buffer, proteins were fractionated by centrifugation into detergent-soluble (S) and -insoluble (P) fractions, and parkin was analyzed by immunoblotting using the anti-parkin antibody PRK8. Control: non-transgenic zebrafish embryos. |