- Title
-
Estrogen receptor ESR1 controls cell migration by repressing chemokine receptor CXCR4 in the zebrafish posterior lateral line system
- Authors
- Gamba, L., Cubedo, N., Ghysen, A., Lutfalla, G., and Dambly-Chaudière, C.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
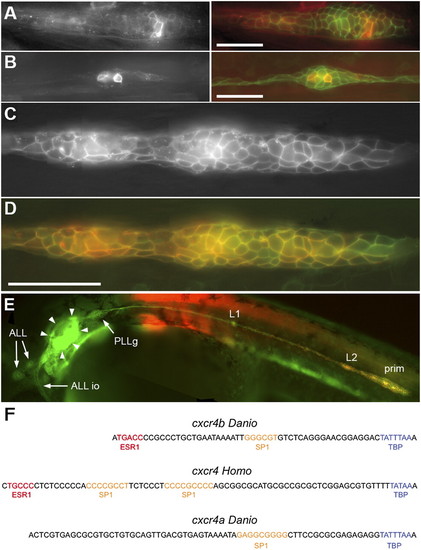
The minimal promoter of cxcr4b. (A and B) transient expression of RFP under the control of the 139-bp cxcr4b promoter fragment in a migrating primordium (A) and in a deposited neuromast (B) in cldnb:gfp embryos. (Left) RFP fluorescence, (Right) RFP in red, merged with GFP in green. (C) Stable expression of RFP driven by the 139-bp promoter in a line doubly transgenic for cxcr4b:rfp and cldnb:gfp. (D) Coexpression of RFP (red) and GFP (green) in the same embryo. (C and D) Assembled from four consecutive frames of a Z-stack; the slight mismatch between green and red, in some cells, is due to migration during the imaging process. In this and all other figures anterior is to the left, and the primordium is migrating to the right. (Scale bars: 50 μm.) (E) Low-scale view of an embryo doubly transgenic for cxcr4b:rfp and cldnb:gfp, illustrating the complete absence of RFP expression in anterior lateral line neuromasts (ALL) and in the infraorbital anterior lateral line primordium (ALL io) as well as in the otic system (arrowheads). Faint RFP fluorescence can be detected in the PLL ganglion (PLLg). (F) Comparison of the sequences upstream of the TATA box in Danio cxcr4b, Homo cxcr4, and Danio cxcr4a promoters. The 139-bp cxcr4b minimal promoter defined by us begins one base upstream of the ESR1 putative binding site and includes the initiation ATG; the 157-bp minimal promoter defined in Homo (15) begins 10 bases upstream of the ESR1 binding site and does not include the initiation ATG. The corresponding sequence in the promoter of cxcr4a is also shown. Putative binding sites according the TESS software are colored. The ESR1 binding sites in cxcr4b and human cxcr4 (red) are both followed by a C, consistent with the presence of a pyrimidine at this position in the consensus binding site. The binding sites for SP1 and TATA Binding Protein have been shown to be instrumental for cxcr4 expression in the minimal human promoter (26). EXPRESSION / LABELING:
|
|
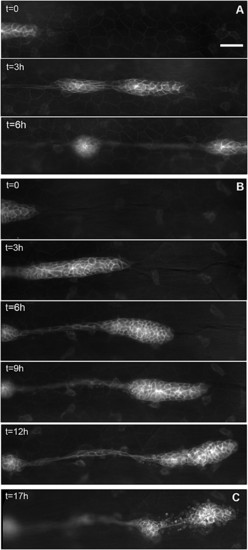
Migration of the PLL primordium is altered in esr1-MO embryos. (A) Three frames of a time-lapse movie of primordium migration in a cldnb:gfp embryo at 35 (t = 0), 38, and 41 hpf, respectively. At 41 hpf, the primordium has just migrated out of the field. (B) Five frames of a time-lapse movie of the primordium in an esr1-MO1 injected embryo at 35 (t = 0), 38, 41, 44, and 48 hpf, respectively. Migration is two to three times slower than in untreated cldnb:gfp embryos and eventually comes to a halt around 45 hpf. (C) In the same embryo, the immobile primordium has fragmented into two neuromasts at 51 hpf. (Scale bar in A: 50 μm.) |
|
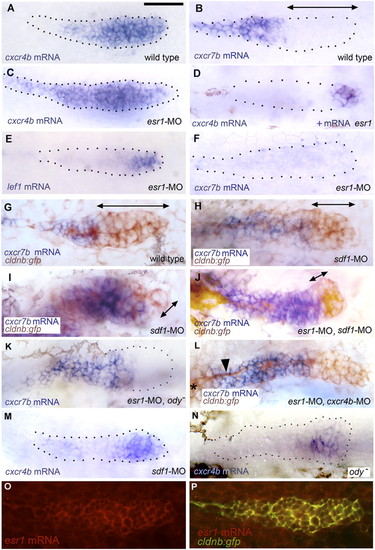
Patterns of gene expression in the PLL primordium. (A and B) Expression of cxcr4b and cxcr7b in 32-hpf control embryos. (C and D) Expression of cxcr4b in conditions of esr1 loss-of-function (C) and gain-of-function (D). The change in expression between A and C was observed in 75% of the injected embryos, respectively, the change between A and D in 30% of the injected embryos. (E and F) Expression of lef1 and cxcr7b in esr1 loss-of-function conditions. (G and H) Expression of cxcr7b (blue) and of gfp (brown) in a 32-hpf control cldnb:gfp embryo (G), in an sdf1-MO embryo (H; an extreme case of cxcr7b domain expansion is shown in I). (J–L) Rescue of cxcr7b expression in esr1-MO embryos that are also sdf1-MO (J), mutant for ody (K), or morphant for cxcr4b (L; note the nerve, arrowhead, extending from the ganglion, asterisk at the left of the figure, under the stalled primordium). (M and N) Expression of cxcr4b in 32-hpf sdf1-MO injected (M) or ody - (N) homozygous embryos; the photograph in N was taken under Nomarski optics to illustrate the extent of the primordium. (O and P) Expression of esr1 in a 32-hpf migrating primordium as revealed by fluorescent in situ hybridization using a rhodamine-coupled antidioxygenin antibody (O) and coexpression of esr1 and cldnb:gfp in the same embryo (P; confocal imaging). (A–F, K, and M) are bright-field pictures without Nomarski optics, to facilitate the detection of expression patterns. The primordia are outlined by dotted lines, based on images taken under Nomarski optics as illustrated in N. Double-headed arrows indicate the extent of the region where cxcr7b expression is not detectable. |
|
Effect of ESR1 on cxcr4b expression. (A) Repression of cxcr4b in all but the leading cells, in embryos injected with esr1 mRNA; the leading cells appear to move ventrally away from the horizontal myoseptum. (B) Complete extinction of cxcr4b expression after injection of ESR1 mRNA; the picture has been taken under Nomarski optics and contrast reinforced to outline the primordium. |
|
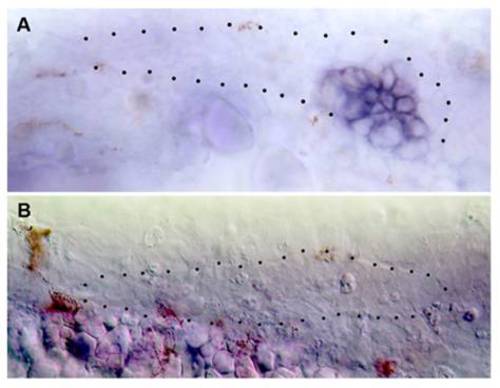
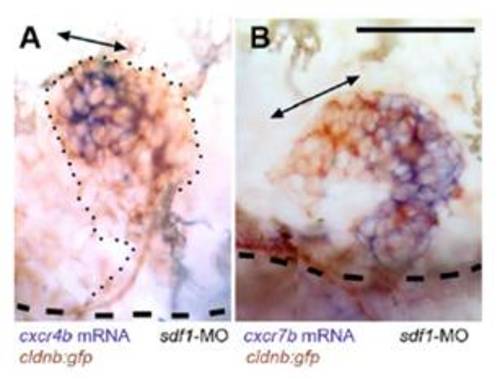
Expression of cxcr4b and cxcr7b in the absence of SDF1/CXCR4 signaling, in two cases of sdf1-MO injected cldnb:gfp embryos where the primordium has left the horizontal myoseptum to migrate dorsally. Double-headed arrows indicate the region of high cxcr4b expression in (A) and of low cxcr7b expression in (B). The dashed lines indicate the position of the horizontal myoseptum. (Scale bar in A: 50 μm.) |
|
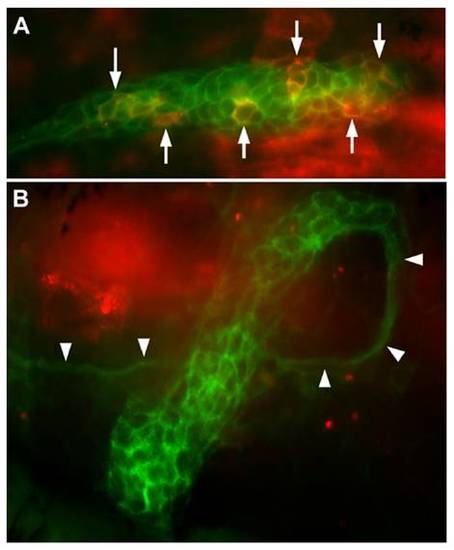
Transient expression driven by the 250-bp promoter in cldnb:gfp embryos (A) and in cldnb:gfp embryos injected with esr1 mRNA (B). A dozen cells are strongly fluorescent in A (arrows), and another 15 are more weakly fluorescent, as observed in half of the injected embryos. No cells are fluorescent in B, as observed in 85% of the injected embryos (see main text). Note the ectopic course of the primordium revealed by the nerve that follows primordium migration (arrowheads): the primordium first followed a normal course; then doubled dorsally and eventually U-turned to dive ventrally. |