- Title
-
Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor
- Authors
- Jing, L., Lefebvre, J.L., Gordon, L.R., and Granato, M.
- Source
- Full text @ Neuron
|
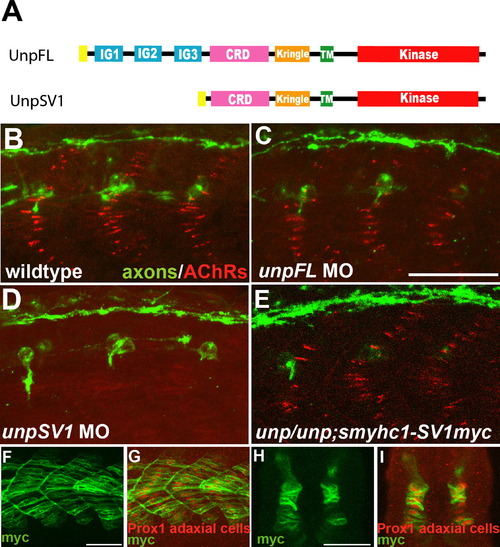
UnpSV1 Controls AChR Prepatterning (A) Domain structure of the Unplugged protein isoforms. (B–E) Lateral views of caudal segments in 17 hpf embryos stained for motor axons (green, znp-1/SV2) and AChRs (red, α-BTX). (B) In wild-type embryos, AChRs are prepatterned in a central band along the dorsal and ventral myotome before the first growth cones approach. (C) UnpFL MO injection does not affect AChR prepattern. (D) UnpSV1 MO injection causes complete absence of AChR prepattern. (E) UnpSV1 expression in adaxial cells restores AChR prepattern in unplugged mutant embryos. (F and G) Lateral views and cross-sectional views (H and I) of 17 hpf Tg(smyhc1:UnpSV1myc) embryos stained with anti-myc (green) and anti-Prox1 (red), which labels the nuclei of adaxial cells. Scale bars, 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
unplugged Restricts Navigating Growth Cones to a Central Muscle Zone (A–D) Still images from time-lapse movies showing the initial migration of single CaP axons (A and B) or CaP/VaP pair axons (C and D) from the spinal cord into the myotome. Arrows point to the single wild-type CaP growth cone (A) and to the tightly fasciculated wild-type CaP/VaP growth cones (C). In contrast, unplugged CaP neurons form extensive filopodia and even multiple growth cones (arrowheads) that occupy a broader area (brackets, [B]). Similary, mutant CaP/VaP growth cones appear defasciculated and occupy a broader area compared to wild-type. Asterisks indicate interneurons also labeled by the Tg(Hb9:GFP) transgene. EXPRESSION / LABELING:
PHENOTYPE:
|
|
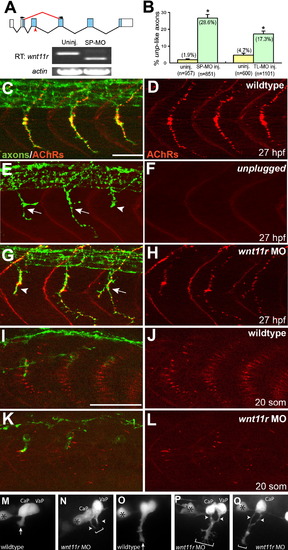
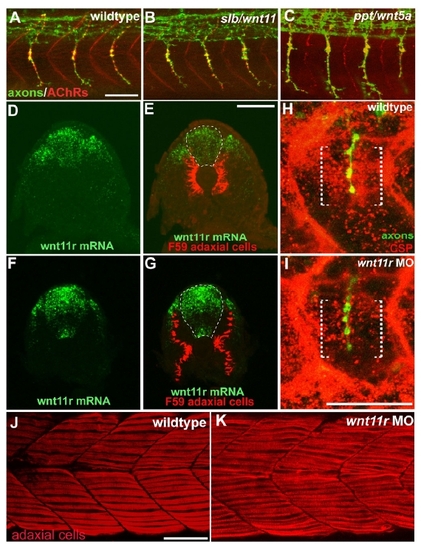
wnt11r Is Critical for Axonal Guidance and AChR Prepatterning (A) The splice morpholino (SP-MO) targets the splice donor site of the wnt11r exon 3 (red arrow), and MO-induced aberrant splicing is shown in red. RT-PCR analyses of uninjected and wnt11r SP-MO injected embryos (arrows indicate the position of PCR primers). (B) Quantification of wnt11r MOs injected embryos. TL-MO, translation initiation mopholino. Per embryo, 20 hemisegments were analyzed; n = hemisegments. Results are expressed as the mean of multiple injection experiments ± SEM, (*p < 0.001, t test). (C–L) Wild-type, unplugged, and wnt11r MO injected embryos at 27 hpf (C–H) and at the 20 somite stage (I–L), stained for motor axons (znp-1, green) and AChR clusters (α-BTX, red). (E and F) In contrast to wild-type, unplugged embryos display characteristic stalling (arrowhead) and branches (arrows) at the choice point and lack all AChR clusters. (G and H) Injection of wnt11r MO causes unplugged-like axonal stalling (arrowhead), branching (arrow), and a strong reduction of AChR prepatterning (K and L). Note that the size and intensity of neural AChR clusters is reduced in wnt11r 27 hpf morphants (H). (M–Q) Time-lapse images of Hb9-GFP-labeled wild-type (M and O) and wnt11r morphant CaP and VaP axons (N, P, and Q) as they exit from the spinal cord (M and N) and as they reach the somitic choice point (O–Q). Asterisks indicate the cell body of interneurons. (M and O) Wild-type CaP and VaP neurons extended one growth cone (arrow). Note the broad area (brackets) the two defasciculated wnt11r morphants CaP/VaP growth cones occupy (arrowheads in [N], [P], and [Q]), compared to wild-type (M and O). Scale bars, 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
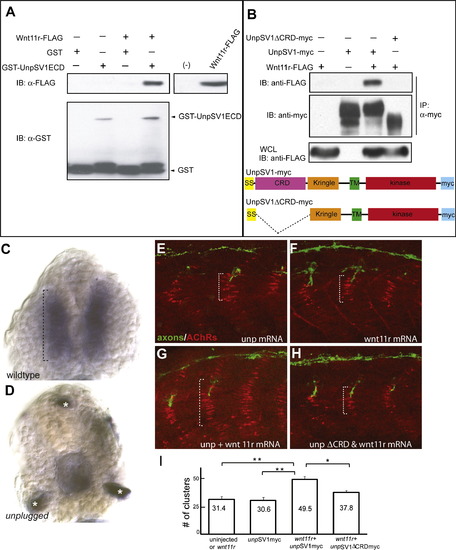
Wnt11r Binds to UnpSV1 and Overepressions of wnt11r and unpSV1 Increase Prepatterning (A) Binding of Wnt11r to the extracellular domain (ECD) of UnpSV1 in vitro. GST-UnpSV1ECD fusion proteins, coupled to glutathione sepharose, were mixed with conditioned media containing secreted Wnt11r-FLAG. Amounts of GST-UnpSV1 and Wnt11r-FLAG used in the analysis were assessed by anti-GST (lower panel) and anti-FLAG (right panel) immunoblotting (IB), respectively. Amounts of Wnt11r-FLAG bound were evaluated by anti-FLAG IB (upper panel). (B) Coimmunoprecipitation of UnpSV1 with Wnt11r in 293T cells. 293T cells were cotransfected with Wnt11r-FLAG and UnpSV1-myc or its CRD deletion mutant. Whole-cell lysates (WCL) were subjected to anti-FLAG IB to determine the expression of Wnt11r-FLAG (lower panel). The UnpSV1-bound Wnt11r was assessed by IB of the anti-myc immunoprecipitate (upper panel). Schematic diagrams of constructs used in the experiments. SS, signal sequence. (C and D) Cross-sections of 20 somite stage embryos injected with purified Wnt11r-FLAG protein. (C) In wild-type embryos, Wnt11r binds to adaxial cells as highlighted by the brackets. Binding is abolished in unplugged mutants(asterisks in [D] mark nonspecific staining). (E–H) Wild-type embryos were injected with mRNAs as indicated. The domain of AChR prepatterning (brackets) was expanded in embryos coinjected with wnt11r and unpSV1myc mRNAs and was dependent on the CRD domain (G and H). (I) Co-overexpression of wnt11r and unpSV1 significantly increases the number of prepatterned clusters/hemisegment (n = 5–18 hemisegments per bar, average = 10). Results are expressed as the average of different injection experiments (t test, **p < 0.01, *p < 0.05). Amounts of mRNA (ng/embryo): wnt11r-FLAG, 0.3; SV1myc, 0.5; SV1ΔCRDmyc, 0.5. AChR cluster size distribution was not altered. |
|
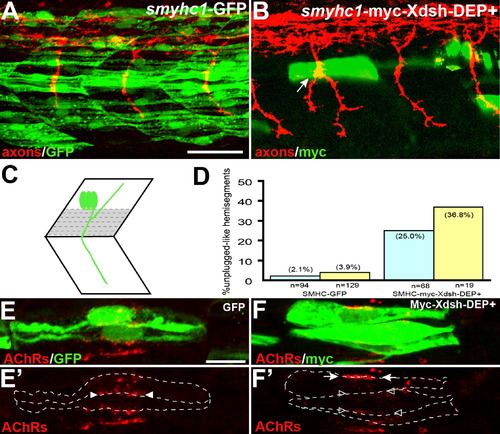
Inhibition of the Noncanonical Dsh Pathway in Adaxial Fibers (A) Stochastic expression of Tg(smyhc1:GFP) in adaxial muscle (green) does not affect motor axons (red). (B) Expression of Tg(smyhc1:myc-XDsh-DEP+) (green) in adaxial fibers dorsal to the choice point causes unplugged-like pathfinding defects (arrow). (C) Location of the dorsal six or seven adaxial cells (in gray) used for scoring. (D) Analysis of axonal phenotypes. n, hemisegments; blue, hemisegments with two adaxial cells expressing the transgene; yellow, hemisegments with three or more adaxial cells expressing the transgene. (E–F′) Confocal images of adjacent adaxial muscle pioneers expressing the smyhc1-GFP or smyhc1-myc-Xdsh-DEP+ transgene. Only AChR clusters between two adjacent transgene-positive adaxial cells were analyzed (outlined by dashed lines). Tg(smyhc1:GFP) expressing adaxial cells form AChR clusters (arrowheads in [E′]), while Tg(smyhc1:myc-XDsh-DEP+ expression disrupts AChR clusters between transgene expressing cells ([F′], open arrowhead); note that this does not affect adjacent, nontransgenic cells that formed normal AChR clusters ([F′], arrows). For each transgene, four embryos with GFP or Myc-Dsh-DEP+ positive adaxial cells were analyzed. Prepatterned clusters were reduced in all Myc-Dsh-DEP+ expressing embryos. Scale bars: (A) 50 μm, (E) 10 μm. |
|
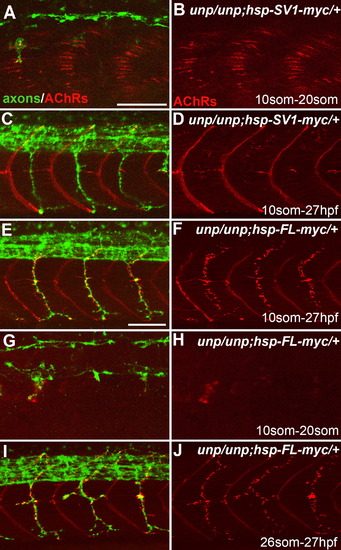
Neuromuscular Synapses Form in the Absence of AChR Prepattern Twenty-somite stage (A, B, G, and H) or 27 hpf (C–F, I, and J) embryos after heat-shock treatment. (A and B) Tg(hsp70l:UnpSV1-myc;unplugged) embryos received heat shock from the 10 to 20 somite stage, which rescued AChR prepattern. (C and D) Similar heat-shock treatment (10 somite to 27 hpf) also restored motor axon pathfinding, but not neuromuscular synapses. (E and F) The same heat-shock treatment rescued motor axons and neuromuscular synapses in Tg(hsp70l:UnpFL-myc; unplugged) embryos. (G and H) In contrast, heat shock between the 10 and 20 somite stage failed to rescue AChR prepattern in Tg(hsp70l:UnpFL-myc;unplugged) embryos. (I and J) Heat-shock treatment of same embryos between the 26 somite stage and 27 hpf, i.e., after the time period of prepatterning, was sufficient to rescue neuromuscular synapses. Scale bars, 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
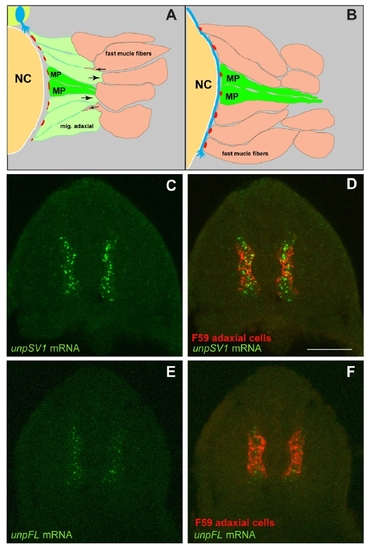
Unplugged full-length (FL) and Splice Variant 1 (SV1) are differentially expressed. |
|
wnt5/11 mutant analysis, wnt11r expression and stainings of wnt11r morphants. |
|
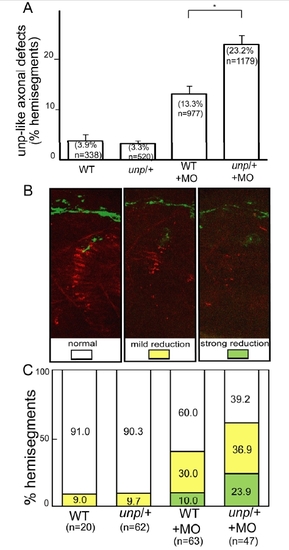
Genetic interactions between unplugged and wnt11r. |
|
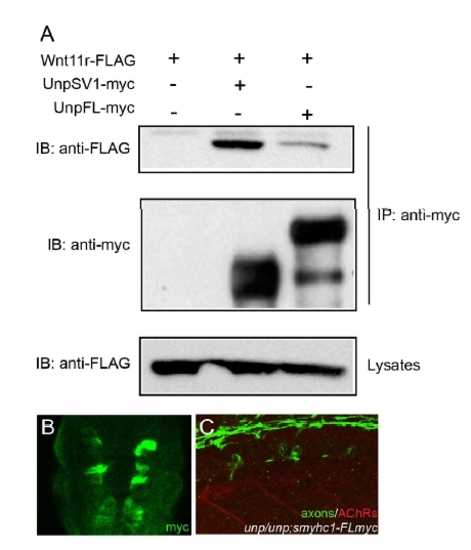
Coimmunoprecipitation of UnpFL with Wnt11r in 293T cells and analysis of the Tg(smyhc1:UnpFLmyc) embryos. (A) 293T cells were cotransfected with Wnt11r-FLAG and UnpSV1-myc or UnpFL-myc. Whole cell lysates (WCL) were subjected to anti-FLAG immunoblotting (IB) to determine the expression of Wnt11r-FLAG (lower panel). The Wnt11r-FLAG binding was assessed by IB of the antimyc immunoprecipitate (upper panel). The amount of UnpFL or UnpSV1proteins were examined by anti-myc western blotting. Wnt11r-FLAG protein coimmunoprecipitated significantly better with UnpSV1 compared to UnpFL. (B) Cross-sectional view of a 17 hpf Tg embryo (smhyc1:unpFLmyc) stained with anti-myc. (C) Expression of UnpFL in adaxial cells failed to restore prepatterned AChRs (red) in unplugged embryos. |
|
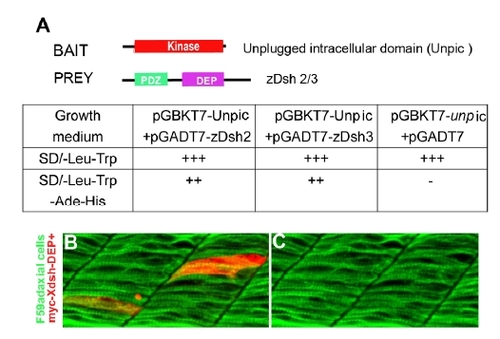
Unplugged interacts with zebrafish Dishevelled. |
|
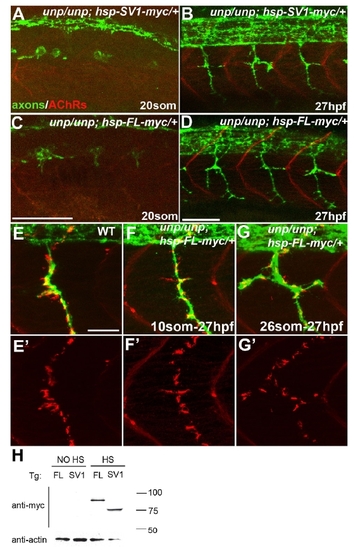
Formation of neural synapses can occur independent of AChR prepatterning. |