- Title
-
Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning
- Authors
- Nissen, R.M., Yan, J., Amsterdam, A., Hopkins, N., and Burgess, S.M.
- Source
- Full text @ Development
|
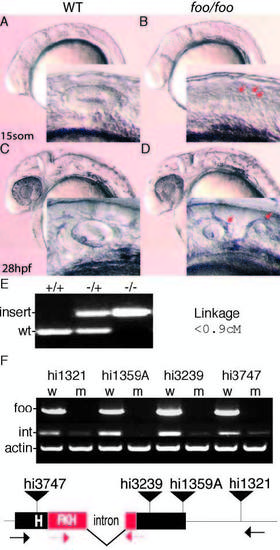
Mutations in zebrafish foxi one cause defects in otic vesicle formation. (A) Lateral view of a wild-type embryo at 15 somites; inset is a magnification of the otic placode. (B) foo/foo embryo at 15 somites; arrows indicate putative placodes. (C) Wild-type ear at 28 hpf. (D) foo/foo embryo at 28 hpf; otic vesicle is clearly split into two smaller vesicles; arrows in inset indicate the two visible vesicles. (E) PCR products from genomic DNA indicating wild-type and mutant alleles of foo with the results of the linkage analysis in centimorgans. (F) RT-PCR analysis of each allele was performed. Wild-type, w; mutant, m. Bands marked foo use primers indicated by black arrows below; bands marked ‘int’ use primers indicated by red arrows. Integration for each allele is marked by a black triangle. The intron and the forkhead (FKH)-related DNA-binding domain are also indicated. PHENOTYPE:
|
|
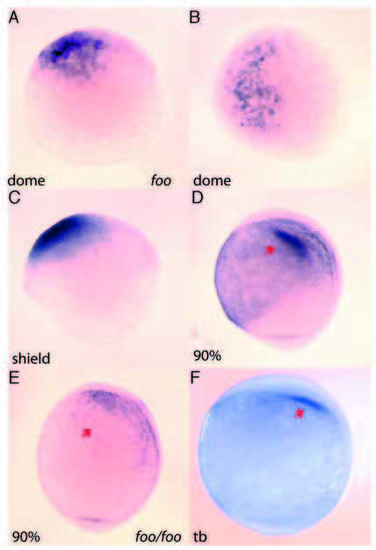
Early expression of foxi one in the developing embryo. (A,B) Antisense RNA in situ hybridization of foo mRNA is first detected in the dome stage of embryos. (A) Lateral view; (B) dorsal view. (C) Lateral view of foo expression at the shield stage. (D) Lateral view 90% epiboly. Arrow indicates foo expression in the otic placode precursors. (E) Lateral view of a foohi3239/foohi3239 embryo, 90% epiboly. Arrow indicates loss of foo expression in the mutant embryo. (F) Lateral view tailbud stage; arrow indicates otic placode precursors expressing foo. |
|
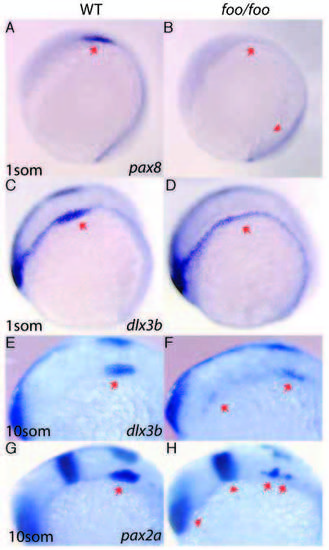
Effects of the foxi one mutation on otic placode markers. Right panels, foo/foo homozygote embryos; left panels, same-clutch wild-type siblings. (A,B) Lateral view of pax8 expression in wildtype (A) and foo/foo (B) one-somite stage embryos. Arrows indicate otic placode region. Arrowhead (B) indicates normal presumptive kidney staining. (C,D) Lateral view of dlx3b staining in one-somite stage wild-type (C) and a foo/foo (D) embryos. Note only otic placode expression is altered in the mutant. (E,F) dlx3b staining in 10-somite stage wild-type (E) and foo/foo (F) embryos. Arrow in E indicates otic placode. Arrows in F indicate scattered staining. (G,H) pax2a expression in 10-somite stage wild-type (G) and foo/foo (H). Arrows indicate scattered otic placode expression in mutant. Arrowheads indicate normal mid-hindbrain boundary and eye expression. EXPRESSION / LABELING:
|
|
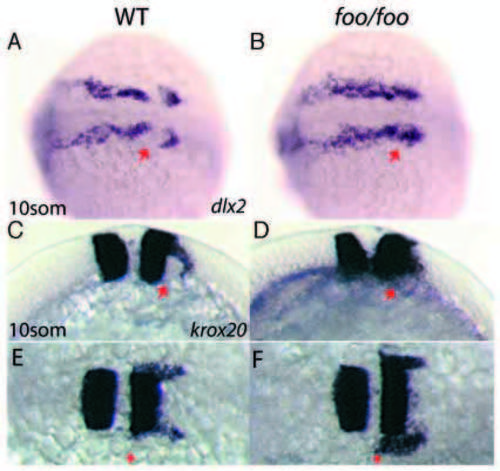
foxi one mutants display defects in third stream neural crest migration. Right panels, foo/foo homozygote embryos; left panels, same-clutch wild-type siblings. (A,B) Dorsal view of dlx2 expression in wild-type (A) and foo/foo (B) 10-somite stage embryos. Arrow in A indicates third stream NC cell migration avoiding the otic placode territory. Arrow in B indicates third stream NC cell invasion of the otic placode territory. (C,E) Lateral (C) and dorsal (E) views of krox20 staining in a 10-somite stage wild-type embryo. Arrows indicate NC cells streaming in a posterior and lateral direction. (D,F) Lateral (D) and dorsal (F) views of a 10-somite stage foo/foo embryo. Arrow in D indicates NC cells invading otic placode territory. Arrow in F indicates approximately normal anterior border for krox20+ NC cells. EXPRESSION / LABELING:
PHENOTYPE:
|
|
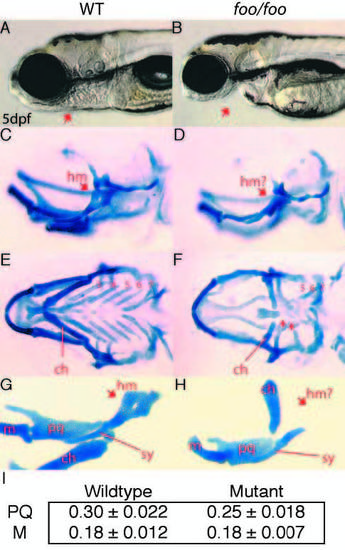
foo mutants display defects in cartilages of the second, third and fourth pharyngeal arches. (A,B) Lateral views of wild-type (A) and foo/foo (B) embryos at 5 dpf. Arrow indicates jaw defects. (C,D) Lateral views of wild-type (C) and foo/foo (D) embryos at 5 dpf; wholemounts, Alcian Blue stained cranial cartilages. Arrow in D indicates the reduced hyomandibular region (hm) of the hyosymplectic. (E) Ventral view of C. The ceratohyal (ch) is indicated and gill arches are numbered 3-7. (F) Ventral view of D. The mildly reduced ch is indicated and arrows indicate the severely reduced third and fourth arch cartilages. The remaining gill arches 5- 7 are indicated. (G) Flatmount of animal in C. Arrow indicates the hm region; line indicates the symplectic rod region (sy). The palatoquadrate (pq), Meckel’s (m) and ch are also indicated. (H) Flatmount of animal in D. Arrow indicates the reduced hm while a line indicates the relatively normal sy. In foo/foo flatmounts, the ch positions abnormally because of an apparent defect in the articulation between ch and the hyosymplectic. (I) Chart comparing the measured length of the palatoquadrate (PQ) and Meckel’s (M) cartilage in wild-type and foo mutant embryos. Measurements were made of eight wild-type and six mutant embryos. The palatoquadrate shows a small but measurable reduction in size in foo mutants. PHENOTYPE:
|
|
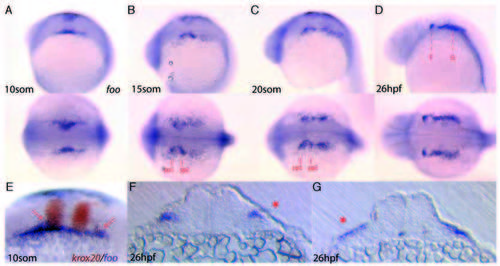
Expression of foo at later stages of development. Detection of foo transcripts in later stages of wild-type embryos. Expression is consistent with both NC cell and future pharyngeal pouch endoderm expression. (A-D) lateral view (top) and dorsal view (bottom) of foo expression at different stages of development. (A) 10- somite stage. (B) 15-somite stage; pharyngeal pouch endoderm of pp1 and pp2 are indicated. (C) 20-somite stage. (D) 26 hpf; broken red lines indicate location of sections shown in F,G. (E) Double in situ hybridization to krox20 and foo in a 10-somite stage embryo. Arrows indicate foo expression in streaming NC cells. (F) Transverse section of a 26 hpf foo in situ hybridized embryo through pp1. Arrow indicates endodermal pouch expression. (G) Transverse section of a 26 hpf foo in situ hybridized embryo through the post-otic region. Arrow indicates foo expression. EXPRESSION / LABELING:
|
|
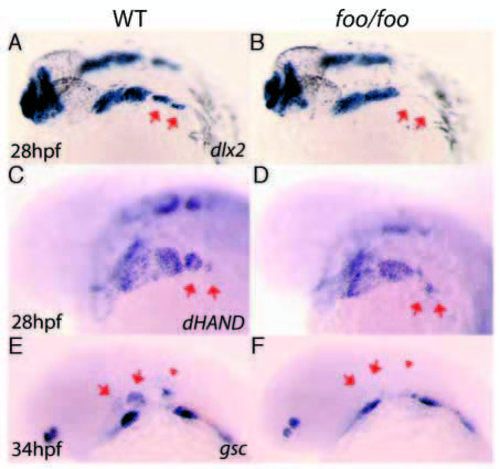
foo mutants show post-migratory NC cell defects. Right panels, foo/foo homozygote embryos; left panels are same-clutch wild-type siblings. (A,B) dlx2 expression by in situ hybridization in wild-type (A) and foo/foo (B) 28 hpf embryos. Arrows indicate third stream derived third and fourth arch NC cells. Note the apparent loss of the third and fourth arch expression domains in the mutant. (C) hand2 expression by in situ hybridization in wild-type (C) and foo/foo (D) 28 hpf embryos. Arrows indicate third stream derived third and fourth arch NC cells. Note the reduction and disorganization of the third and fourth arch expression domains in the mutant. Also note the normal ventral expression of hand2 in the first and second arches. (E,F) gsc expression by in situ hybridization in wild-type (E) and foo/foo (F) 34 hpf embryos. Arrows in E indicate dorsal gsc expression domains in the first and second arch NC cells flanking either side of pp1. Arrowhead in E indicates gsc expression in the otic vesicle. Arrows in F indicate where gsc expression can no longer be seen in the more dorsal region of expression; more ventral domains remain in foo embryos. Otic vesicle expression is also absent (arrowhead in F). |
|
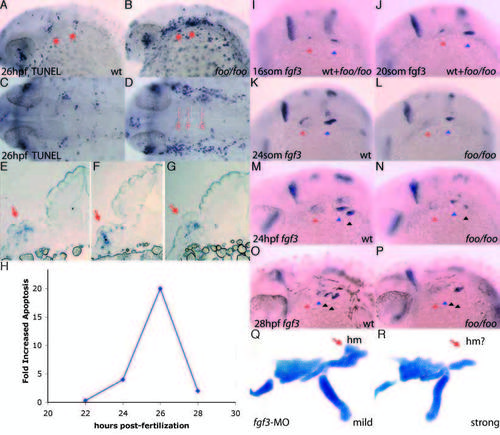
foo mutants show a loss of fgf3 expression maintenance in the pharyngeal pouches and increased apoptosis. (A,B) TUNEL assay on a wild-type (A) and foo/foo (B) embryos at 28 hpf, lateral views. Arrows indicate pp1 and pp2 with increased apoptosis. (C) Dorsal view of A; (D) dorsal view of B. Broken lines indicate approximate section locations. (E-G) Sections of TUNEL assay stained foo/foo embryos indicated in D. Arrows indicate TUNEL-positive cells. (H) Transient increase in apoptosis observed in foo/foo embryos. Quantitation was performed using NIH-Image for densitometry analysis of corresponding arch regions between mutant and wild-type embryos. Data points represent averages for at least three embryos. (I-P) fgf3 expression by in situ hybridization in foo/foo (I,J,L,N,P) and wild-type (I,J,K,M,O) siblings. (I) 16- somite stage (mutants are indistinguishable from wild type at this stage). (J) 20- somite stage (mutants can not be distinguished from wild type). (K) 24-somite stage embryo. fgf3 expression is maintained in pp1 (red arrowhead) and pp2 (blue arrowhead). (L) 24-somite stage. fgf3 expression maintenance is lost from pp1, while pp2 expression is slightly lower than in wild type (K), but clearly visible. (M) 24 hpf embryo. fgf3 expression is maintained in pp2 (blue arrowhead) and pp3 (black arrowhead). (N) 24 hpf embryo. fgf3 expression maintenance is reduced from pp2 and initiation is visible in pp3. (O) 28 hpf embryo. fgf3 expression is absent from pp1 (red arrowhead), is downregulated in pp2 (blue arrowhead), maintained in pp3 (left black arrowhead) and initiated in pp4 (right black arrowhead). (P) 28 hpf embryo. Expression is initiated normally in pp4 (second black arrowhead) but is prematurely lost from pp2 (blue arrowhead) and pp3 (left black arrowhead). (Q) Flat-mounted Alcian Blue staining of a mildly affected 4 dpf embryo injected with 5 ng of fgf3-MO (Phillips et al., 2001). Hyosymplectic (red arrow) appears relatively normal. (R) A more strongly affected 4 dpf embryo injected with 5 ng of fgf3-MO shows a reduction in the hyosymplectic (red arrow) consistent with the skeletal defects observed in foo/foo embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
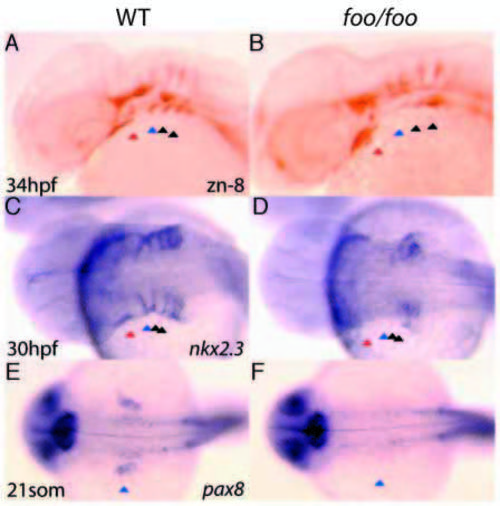
The foo mutation affects pouch formation. Right panels, foo/foo homozygote embryos; left panels, same-clutch wild-type siblings. (A) zn-8 antibody staining of a wild-type embryo at 34 hpf. Pharyngeal pouches (pp1-pp4) are indicated by arrowheads as in Fig. 8. (B) zn-8 antibody staining of a 34 hpf foo/foo embryo. The posterior pouches pp2-4 are substantially reduced, whereas pp1 is only mildly affected. (C) nkx2.3 expression by in situ hybridization of a 30 hpf wild-type embryo. (D) nkx2.3 expression in a 30 hpf foo/foo embryo. Most of the nkx2.3 expression is absent or disorganized. (E) pax8 expression in a 21-somite wild-type embryo with pp2 indicated as in A. (F) pax8 expression in a 21-somite foo/foo embryo. Expression of pax8 in pp2 is absent and the otic vesicle is substantially reduced. EXPRESSION / LABELING:
|