Fig 7
- ID
- ZDB-FIG-200829-99
- Publication
- Wilson et al., 2020 - A point mutation decouples the lipid transfer activities of microsomal triglyceride transfer protein
- Other Figures
- All Figure Page
- Back to All Figure Page
|
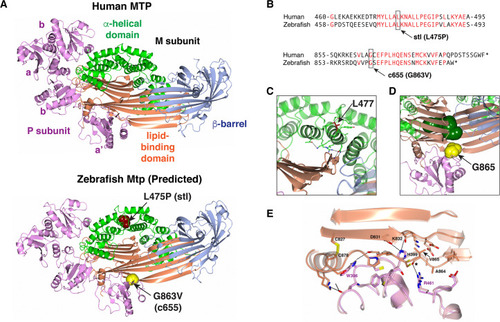
(A) Ribbon representation of the human MTP complex (PDB entry 6I7S) and the Zebrafish modeled structure. The positions of L475 and G863 in the Zebrafish structure are shown in space-filling representation. (B) Alignment of human MTP and zebrafish Mtp amino acid sequences surrounding the |