- Title
-
Characterization of a novel zebrafish model of MTMR5-associated Charcot-Marie-Tooth disease type 4B3
- Authors
- Lindzon, J., List, M., Geissah, S., Ariaz, A., Zhao, M., Dowling, J.J.
- Source
- Full text @ Brain Commun
|
|
|
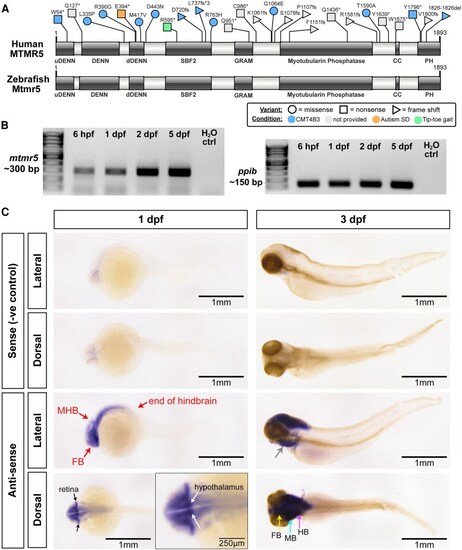
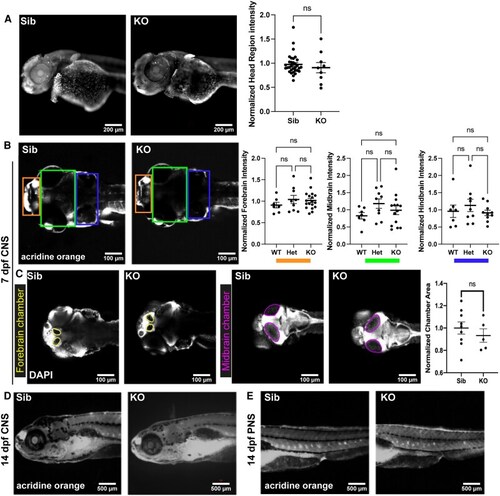
EXPRESSION / LABELING:
|
|
|
|
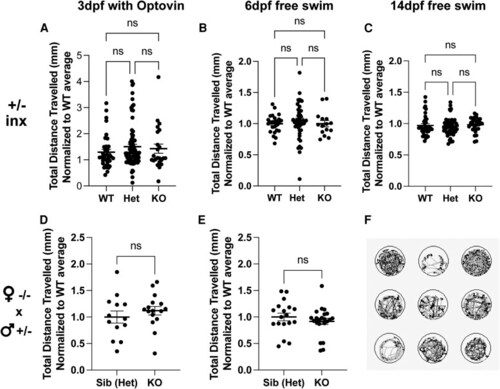
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
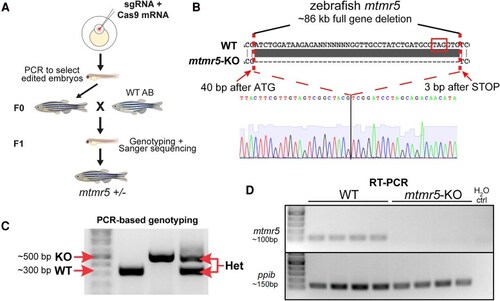
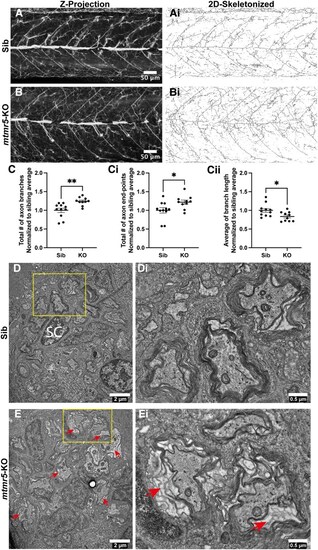
EXPRESSION / LABELING:
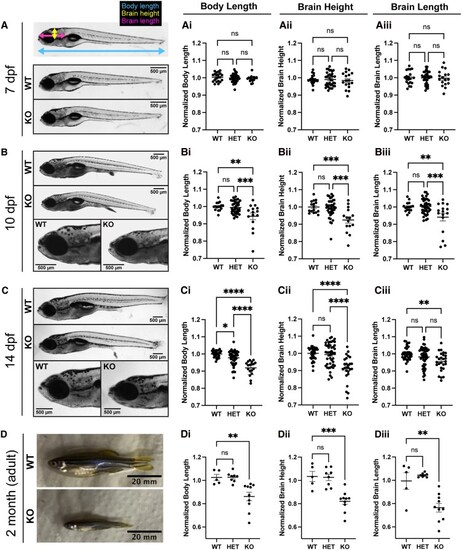
PHENOTYPE:
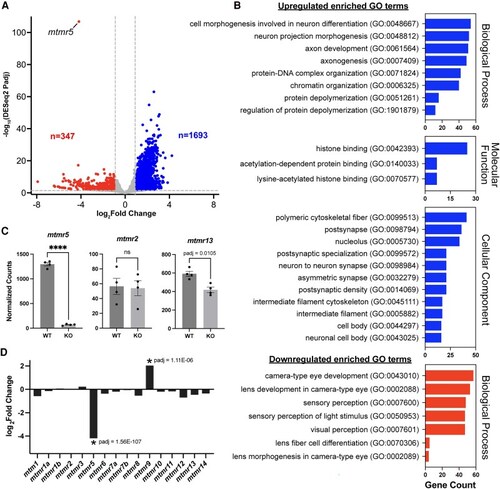
|
|
|