- Title
-
The Serotonergic Dorsal Raphe Promotes Emergence from Propofol Anesthesia in Zebrafish
- Authors
- Yang, X., Zhu, S., Xia, M., Sun, L., Li, S., Xiang, P., Li, F., Deng, Q., Chen, L., Zhang, W., Wang, Y., Li, Q., Lyu, Z., Du, X., Du, J., Yang, Q., Luo, Y.
- Source
- Full text @ J. Neurosci.
|
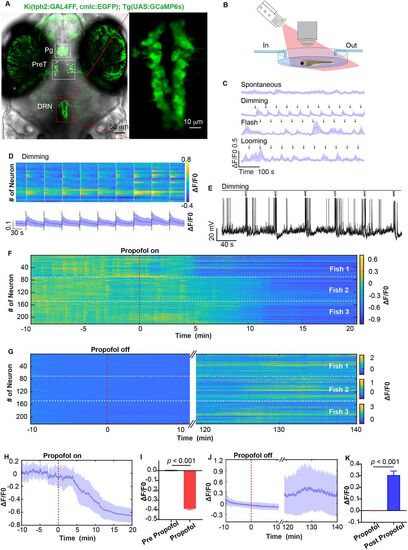
Calcium activity of DRN5-HT neurons were suppressed by propofol. A, The tph2 knock-in mutant line Ki(tph2:GAL4FF,cmlc2:EGFP);Tg(UAS:GCaMP6s) marks 5-HT neuron at pineal gland (Pg), pretectum (PreT), and DRN. B, Propofol was perfused and eluted during in vivo calcium imaging at single cellular resolution, accompanied by visual stimuli by a projector. C, Spontaneous calcium activity of DRN5-HT and calcium activity responded to dimming, flash and looming stimuli. Data represent mean ± SEM. n = 3 larvae. D, Representative calcium activity stimulated by dimming at 58 s interval (top) and the mean trace (bottom) from a layer of DRN5-HT. The dash line indicates dimming stimulation. Data represent mean ± SEM. E, In vivo whole-cell electrophysiology showed not that dimming stimuli did not influence the membrane potential of DRN5-HT. F,G, Heatmaps of calcium activity during perfusion and elution of propofol from 221 DRN5-HT neuron of three larvae. The slow recovery of neural activity is attributed to the slow perfusion speed (1.66 ml/min) to ensure imaging stability and potential quenching of GCaMP6s fluorescence signal over long imaging periods (Extended Data Fig. 1-1). H,I, Calcium events were significantly inhibited by 30 µM propofol perfusion. H, mean ± SEM, indicated by the full line and shaded area. I, Pre-propofol versus propofol; 0 versus −0.390 ± 0.007; p < 0.001; paired t test. J,K, Calcium activity recovered after propofol was eluted. J, Mean ± SEM, indicated by the full line and shaded area. K, Propofol versus post-propofol, 0 versus 0.305 ± 0.035; p < 0.001; paired t test. |
|
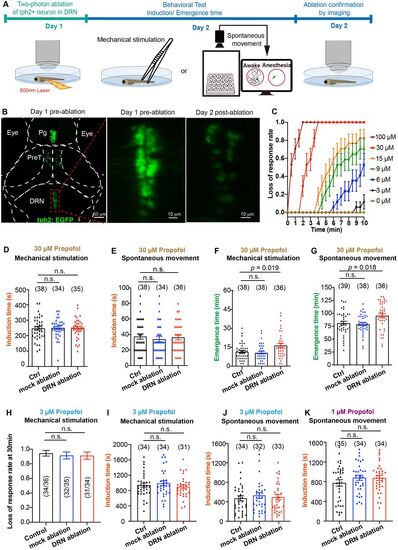
Two-photon laser ablation of DRN5-HT neurons retarded emergence from propofol anesthesia. A, Behavioral experiments diagram. B, The representative images of two-photon laser ablation on DRN5-HT neurons. C, Dose response curve of propofol on the loss-of-response to tail-fin pinch. N = 21 larvae/concentration. D,E, Specific ablation of DRN5-HT had little effect on the induction time of 30 μM propofol anesthesia detected by mechanical stimulation (D, control group, 247.1 ± 12.1 min; mock ablation, 250.6 ± 10.7 min; DRN ablation, 249.4 ± 11.2 min; p > 0.05; one-way ANOVA) and loss of spontaneous movement (E, control group, 37.4 ± 3.4 min; mock ablation, 34.1 ± 3.6 min; DRN ablation, 36.4 ± 3.2 min; p > 0.05; one-way ANOVA). F,G, Specific ablation of DRN5-HT significantly prolonged emergence time from 30 μM propofol, detected by both recovery to mechanical stimulation (F, F = 5.91, control vs DRN ablation; 11.5 ± 1.0 min vs 16.5 ± 1.7 min; p = 0.019; one-way ANOVA) and the recovery time of spontaneous movement (G, F = 5.70; control vs DRN ablation; 81.0 ± 3.7 min vs 95.4 ± 4.2 min; p = 0.036; one-way ANOVA). H–J, Specific ablation of DRN5-HT had little effect on the induction time of 3 μM propofol anesthesia detected by mechanical stimulation (H, loss of response rate at 30 min; I, induction time, control group, 943.2 ± 53.8 s; mock ablation, 990.9 ± 63.6 s; DRN ablation, 900.0 ± 50.6 s; p > 0.05; one-way ANONA) and loss of spontaneous movement (J, control group, 472.6 ± 52.4 s; mock ablation, 495.3 ± 54.1 s; DRN ablation, 530.3 ± 59.9 s; p > 0.05; one-way ANOVA). K, Specific ablation of DRN5-HT had little effect on the induction time of 1 µM propofol anesthesia detected by loss of spontaneous movement (control group, 776.0 ± 64.5 s; mock ablation, 880.6 ± 51.8 s; DRN ablation, 876.2 ± 50.1 s; p > 0.05; one-way ANOVA). The sample size for each group is indicated in parentheses. In H, the values in parentheses are the ratio of the number of larvae that lost response to the total sample size. |
|
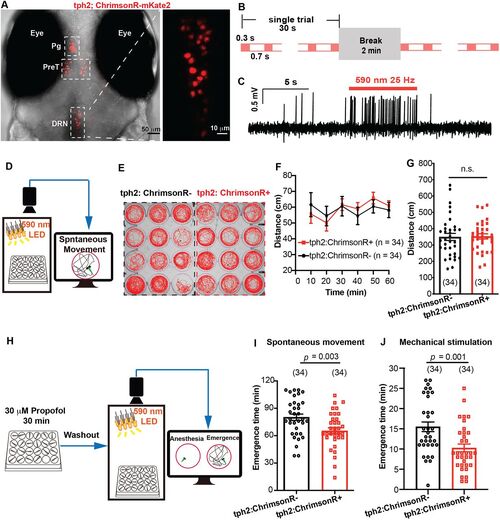
Optogenetic activation of tph2+ neurons promoted emergence from propofol anesthesia. A, The expression of Ki(tph2: GAL4FF,cmlc2:EGFP);Tg(UAS: ChrimsonR-mKate2) mutant line. Pg, pineal gland; PreT, pretectum; DRN, dorsal raphe nucleus. B, Optogenetic stimulation paradigm during the whole process of emergence. C, Confirmation of ChrimsonR activation by in vivo cell-attached recording. D, The diagram of behavior experiment detecting the influence of field optogenetic activation on locomotor activity. E, The spontaneous movement trajectory under optogenetic stimulation during 10 min. F,G, Distance moved under optogenetic stimulation during 1 h and the statistical analysis (tph2, ChrimsonR−, vs tph2, ChrimsonR+; 349.8 ± 21.7 mm vs 353.0 ± 15.8 mm; p > 0.05; unpaired Student's t test). H, The diagram of behavioral experiment detecting the influence of field optogenetic activation on the emergence time from propofol. I,J, Optogenetic stimulation significantly promoted the emergence from propofol anesthesia detected by recovery of spontaneous movement and recovery to mechanical stimulation (spontaneous movement, tph2, ChrimsonR−, vs tph2, ChrimsonR+; 80.2 ± 3.5 min vs 65.3 ± 3.3 min; p = 0.003; mechanical stimulation, tph2, ChrimsonR−, 15.5 ± 1.2 min; tph2, ChrimsonR+, 10.3 ± 1.0 min; p = 0.001; unpaired Student's t test). The sample size for each group is indicated in parentheses. |
|
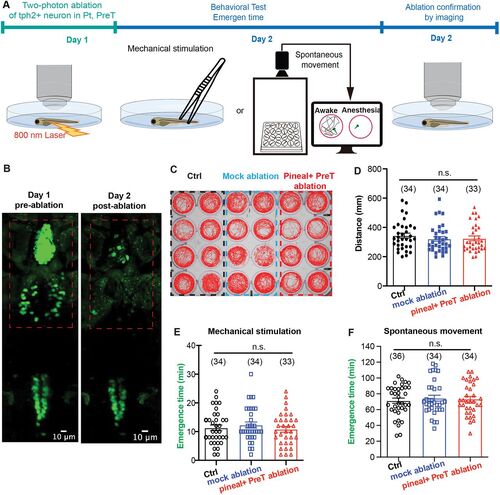
Two-photon laser ablations of tph2+ neurons in pineal and pretectum did not influence the emergence from propofol. A, Behavioral experiments diagram. B, The representative images of two-photon laser ablation on pineal and pretectum. C, The spontaneous movement trajectory of the control group, mock ablation group, and pineal + pretectum ablation group during 10 min. D, Distance moved during 1 h and the statistical analysis (control vs mock ablation vs pineal + preT ablation; 349.8 ± 18.0 mm vs 330.1 ± 17.5 mm vs 336.5 ± 17.7 mm; p > 0.05; unpaired Student's t test). E,F, Two-photon laser ablations of pineal and pretectum did not influence the emergence time from propofol anesthesia, detected by both recovery to mechanical stimulation (E, control, 11.3 ± 1.0 min; mock ablation, 12.3 ± 1.0 min; pineal + PreT ablation, 10.8 ± 1.0 min) and the recovery time of spontaneous movement (F, control, 71.3 ± 3.3 min; mock ablation, 74.5 ± 3.8 min; pineal + PreT ablation, 73.3 ± 3.5 min). p > 0.05; one-way ANOVA. |
|
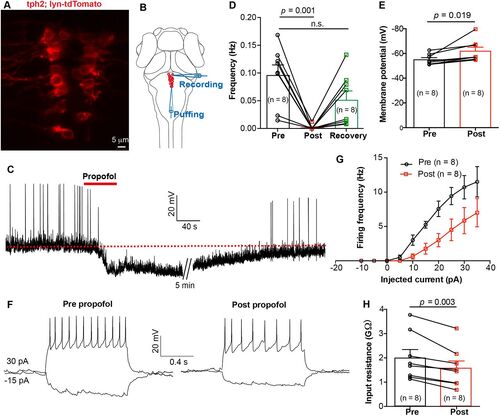
In vivo whole-cell recording revealed that propofol decreased the excitability of DRN5-HT. A, The expression of Ki(tph2:GAL4FF,cmlc2:EGFP);Tg(UAS: lyn-tdTomato) mutant line at dorsal raphe. B, Schematic diagram showing whole-cell recording of DRN5-HT neurons and simultaneous local puffing of propofol onto DRN5-HT. C, Spontaneous spike firing of DRN5-HT neuron before and after puffing of propofol. The red dashed line indicates the mean resting membrane potential before propofol treatment. D,E, Summary of data showing the effect of propofol treatment on the spontaneous spike frequency (D, pre vs post, 0.10 ± 0.02 Hz vs 0.00 ± 0.00 Hz; p = 0.001; pre vs recovery, 0.10 ± 0.02 Hz vs 0.05 ± 0.02 Hz; p > 0.05; paired Student's t test) and resting membrane potential (E, pre vs post, −55.1 ± 1.5 mV vs −62.0 ± 3.1 mV; p = 0.019; paired Student's t test) of DRN5-HT neurons. Pre, before puffing; post, immediately after puffing; recovery, 10 min after puffing. F, Representative traces showing voltage responses of DRN5-HT evoked by current injections before (left) and immediately after (right) puffing of propofol. G, Summary of data showing the effect of propofol on current injection-evoked spike firing of DRN5-HT neurons. p = 0.01, Kolmogorov–Smirnov test. H, Summary of data showing the effect of propofol on the membrane resistance of DRN5-HT neurons. Pre versus post, 2.0 ± 0.3 versus 1.6 ± 0.3 GΩ; p = 0.019; paired Student's t test. Numbers of cells examined are in parentheses. |
|
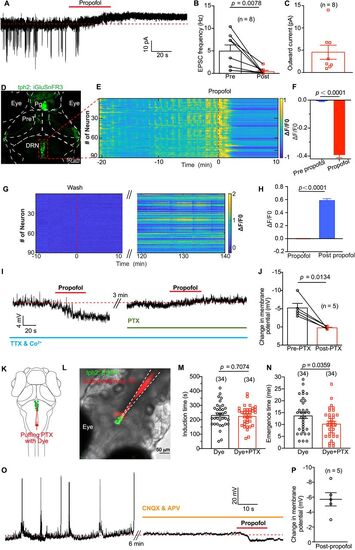
Propofol inhibits presynaptic excitatory glutamate inputs to DRN5-HT and cause membrane hyperpolarization of DRN5-HT. A, Spontaneous EPSCs of a DRN5-HT neuron (clamped at −60 mV) before and after puffing of propofol. The red dashed line indicates the mean leakage current before propofol application. B, Summary of data showing the reduction of EPSC frequency by propofol application. Pre versus post, 4.95 ± 1.36 Hz versus 0.40 ± 0.26 Hz; p = 0.001; paired Student's t test. N = 8 cells from different larvae. C, Summary of data showing the outward current induced by propofol application. N = 8 cells from different larvae. D, Transient expression of glutamate sensor by injecting UAS, iGluSnFR3 plasmid into Ki (tph2, GAL4FF; cmlc2, EGFP) line. Pg, pineal gland; PreT, pretectum; DRN, dorsal raphe nucleus. E, Representative heatmaps of glutamate events from DRN5-HT of one larva stimulated by dimming at 58 s interval. The red dash line indicates the timing of adding propofol to the dish. F, Glutamate events were significantly inhibited by 30 µM propofol. N = 259 DRN5-HT neurons from three larvae. G,H, iGlusnFR3 signal gradually recovered when propofol was washed by perfusion. I, Representative trace showing propofol-induced membrane potential changes of a DRN5-HT neuron before and after application of 100 mM PTX. During the recording, synaptic transmission was blocked by replacing Ca2+ with Co2+ in the extracellular solution and adding 1 mM TTX. J, Summary of data showing the effect of PTX on propofol-induced membrane potential changes. Without PTX versus with PTX, −5.2 ± 1.3 mV versus 0.2 ± 0.2 mV; n = 5; p = 0.013; paired Student's t test. K, Schematic diagram showing local puffing of PTX with the red fluorescent dye sulforhodamine 101. L, A typical case showing the area of puffing PTX with the red fluorescent dye sulforhodamine 101. M, N, Inhibiting GABAA receptors in DRN by local puffing PTX had no influence on induction time of propofol while significantly shortening the emergence time from propofol anesthesia (N, dye vs dye + PTX, 13.8 ± 1.2 min vs 10.3 ± 1.0 min; p = 0.036; unpaired Student's t test). O, P, After blocking glutamatergic receptors by adding 50 µM CNQX and 100 µM APV in the extracellular solution, local puffing of propofol caused a significant hyperpolarization of DRN5-HT (mean ± SEM, −5.7 ± 0.9 mV; n = 5 neuron). Numbers of the sample size are in parentheses. |