- Title
-
A novel DSP zebrafish model reveals training- and drug-induced modulation of arrhythmogenic cardiomyopathy phenotypes
- Authors
- Celeghin, R., Risato, G., Beffagna, G., Cason, M., Bueno Marinas, M., Della Barbera, M., Facchinello, N., Giuliodori, A., Brañas Casas, R., Caichiolo, M., Vettori, A., Grisan, E., Rizzo, S., Dalla Valle, L., Argenton, F., Thiene, G., Tiso, N., Pilichou, K., Basso, C.
- Source
- Full text @ Cell Death Discov
|
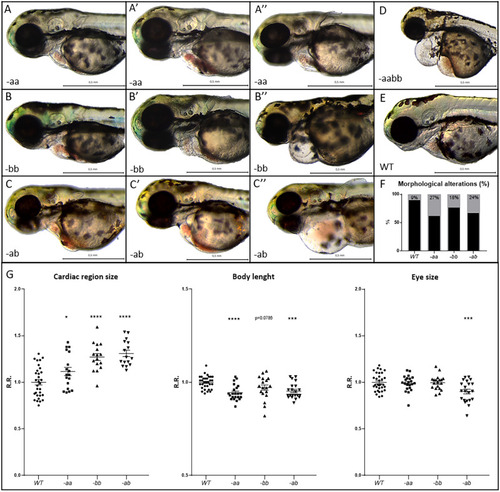
Cardiac alterations and developmental delay in Dsp mutant lines. PHENOTYPE:
|
|
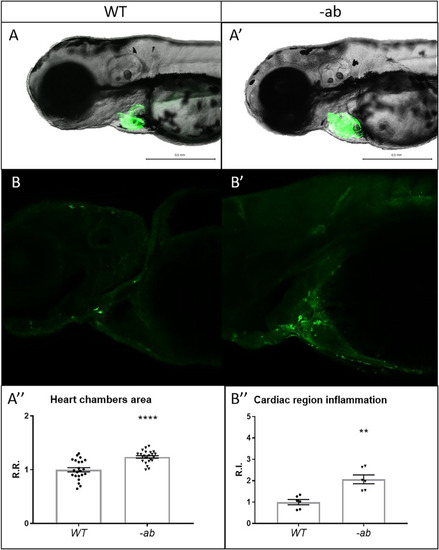
Detection of cardiac dilation and inflammation in zebrafish Dsp mutants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
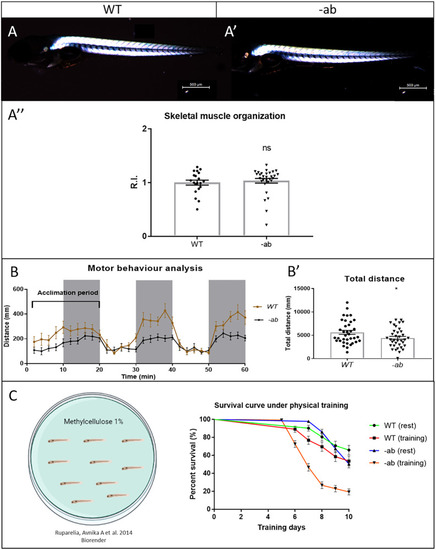
Impaired motor behaviour and exercise-induced mortality in zebrafish Dsp mutant larvae. |
|
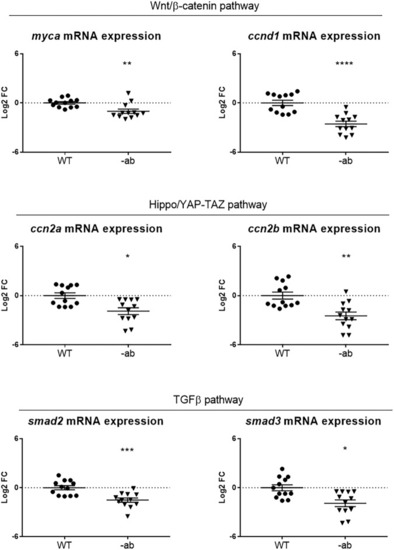
Signalling pathways dysregulation in Dsp mutant adult hearts. qPCR analysis of the expression of Wnt/β-catenin, YAP-TAZ and TGF-β signalling members showed a downregulation of all pathways in 1-year old Dsp mutant hearts. Each point on the graph corresponds to a pool of 3 hearts of the same genotype. Sample size: |
|
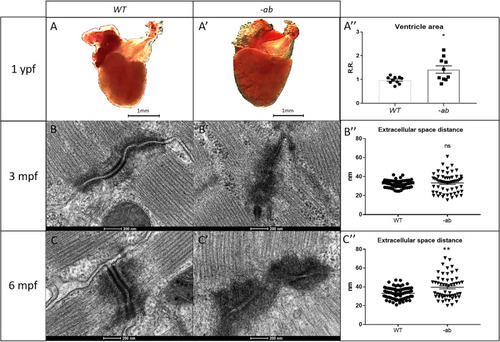
Cardiac dilation and TEM analysis of 3- and 6-month old Dsp mutant zebrafish hearts. PHENOTYPE:
|
|
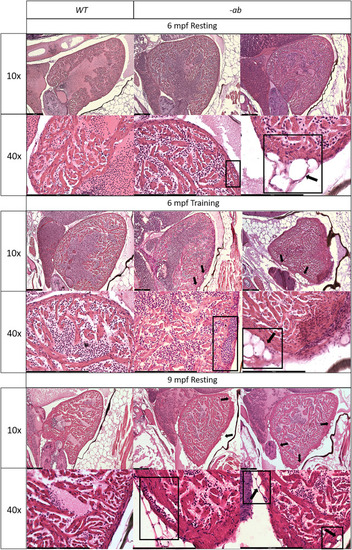
Cardiac dilation and structural changes in Dsp mutant hearts Histological analysis of 6-month old -ab mutant zebrafish showed mild rarefaction of cardiomyocytes, thinning of the myocardial layer, age-related alterations in the distribution and organization of the trabeculae network, an abnormal shape of the ventricle, the presence of possible vessels dilation (rectangular box) and accumulation of adipose cells outside and inside the myocardial layer, in ≈50% of analyzed fish (square boxes and black arrows). Histological analysis of 6-month old mutated zebrafish confirmed the worsening of the condition after intensive physical training, like vessels dilation (rectangular box) and a more intrusive presence of adipose cells, in ≈80% of analyzed fish (square boxes and black arrows), showing similarities with 9-month old mutant hearts at rest. 9-month-old -ab mutant zebrafish showed worsening of the cardiac phenotype compared to 6-month old mutant hearts, with thickness of the myocardial layer, vessels dilation (rectangular box), and more intrusive presence of adipose cells, in ≈80% of analyzed fish (square boxes and black arrows). Sample size: PHENOTYPE:
|
|
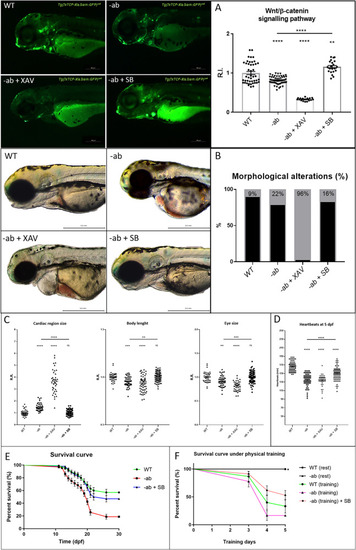
Wnt/β-catenin activation rescues AC phenotypes in Dsp zebrafish mutants. PHENOTYPE:
|