- Title
-
Her6 and Prox1a are novel regulators of photoreceptor regeneration in the zebrafish retina
- Authors
- Veen, K., Krylov, A., Yu, S., He, J., Boyd, P., Hyde, D.R., Mantamadiotis, T., Cheng, L.Y., Jusuf, P.R.
- Source
- Full text @ PLoS Genet.
|
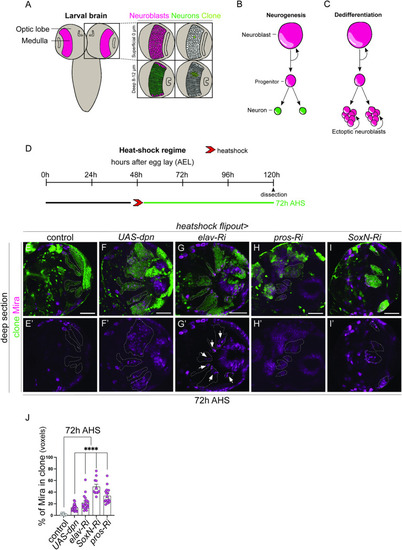
Knockdown of Dpn, Elav, Pros, and SoxN result in dedifferentiation of medulla neurons. (A) Schematic representation of |
|
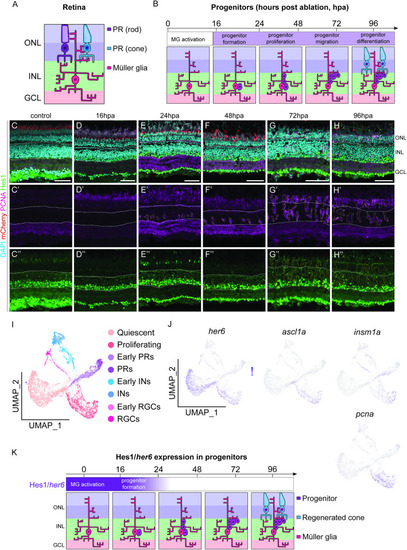
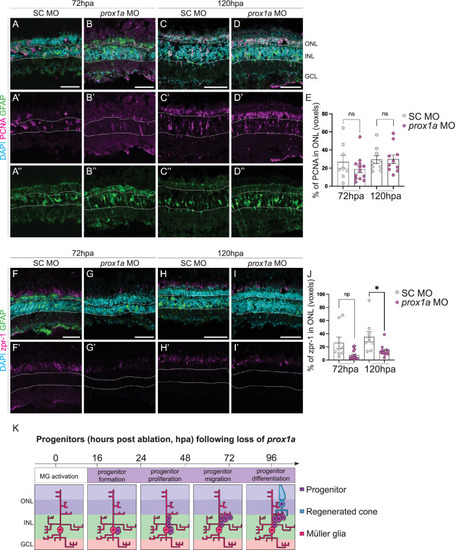
Her6/Hes1 is reduced in regenerating progenitors. (A, B) Schematic representation of retinal cells and observed regenerative processes and timeline. Rod (purple) and cone (blue) photoreceptors (PR) are located in the outer nuclear layer (ONL). Müller glia (MG) are located in the inner nuclear layer (INL) with processes spanning across the retina from the ONL through to the GCL (ganglion cell layer). Following PR ablation, MG are activated and dedifferentiate into progenitors, which proliferate and migrate prior to differentiation into photoreceptors by 96 hours post ablation (hpa). (C-H”) In adult retina, during the 96-hour regenerative time span following the ablation of PRs (red), proliferation is marked by proliferative cell nuclear antigen (PCNA, pink) starts at 24 hpa. Expression of Hairy-enhancer of split (Hes1, green) is seen both in the control and following the ablation in the inner half of the INL and in the GCL. Focusing on the proliferative cells, the expression of Hes1 is downregulated as PCNA expression increases from 24 hpa onwards. DAPI (cyan) marks the retinal layers. Scale bars: 50 μm.(I) UMAP plot of FACS MG 3 days after injury is subdivided into distinct MG derived cell populations based on stereotypical markers. (J) The feature plots show that |
|
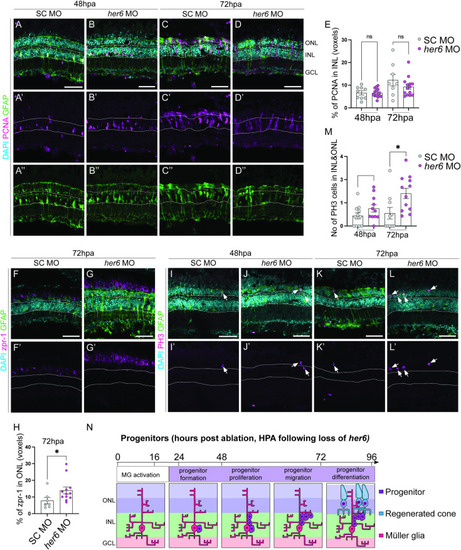
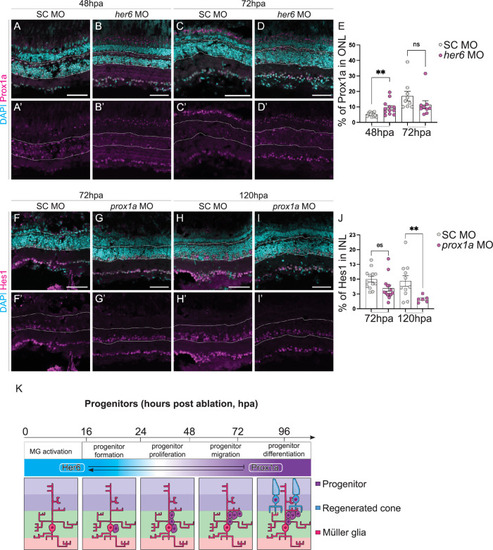
Loss of Her6 increases PR production at 72 hours post ablation. (A-D”) In adult retina, at 48 and 72 hours post ablation (hpa), the number of proliferating cells marked by PCNA (pink) and Müller glia (MG) cells marked by glial fibrillary acidic protein transgene expressing (GFAP, green) are not significantly altered between standard control (SC, 48hpa n = 9, 6.621 ±0.9034, 72hpa n = 8, m = 12.27 ±2.579) and |
|
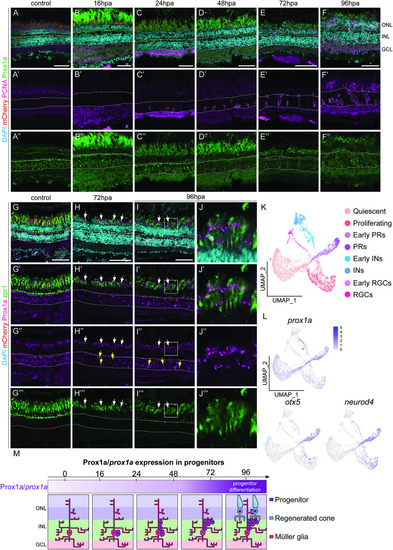
Prox1a is expressed in the photoreceptors within the outer nuclear layer. (A-F”) Within adult retinas in uninjured control, post ablation (hpa), Prospero homeobox1a (Prox1a, green) is expressed in the inner nuclear layer (solid white outline), but never in proliferating (proliferative cell nuclear antigen positive, PCNA, pink) cells (dotted outlines). At 72hpa and 96hpa Prox1a in addition expressed in the ONL. (G-I”) cone photoreceptor marker, zpr1 (green) is expressed in the ONL in the control and at all the time points post ablation. (J-J”’) is the magnified images of (I-I”’). At 72hpa and 96hpa, Prox1a is expressed as distinct puncta within zpr1 expressing photoreceptors (arrows and high-power inset). Scale bars: 50 μm. (K, L) UMAP plot of FACS MG 3 days after injury can be subdivided into distinct MG derived cell population based on stereotypical markers. The feature plots show that |
|
Loss of Prox1a decreases PR production at 120 hours post-ablation. (A-D”) Knockdown of Prox1a via morpholino (MO) in adult retina during regeneration does not significantly affect percentage of PCNA+ (pink) proliferative Müller glia (marked by GFAP, green) following |
|
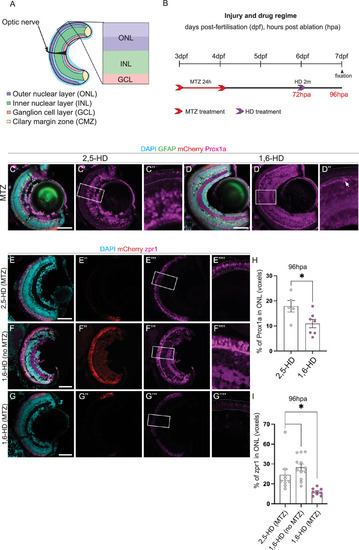
Prox1a puncta in liquid-liquid phase separates regulate PR differentiation. (A) Schematic highlighting the retinal layers analysed in the zebrafish larvae. (B) Experimental timeline. Larvae were swum in metronidazole (MTZ) for 24 hours at 3dpf (days post-fertilisation), followed by a 2 min treatment with 1,6-Hexanediol (1,6-HD) or 2,5-HD at 6dpf, before processing at 7dpf. (C-D”) Following photoreceptor (PR) ablation, Tg( |
|
Her6 reduction increases Prox1a expression. (A-D’) Downregulation of |