- Title
-
Genomic disturbance of vitellogenin 2 (vtg2) leads to vitellin membrane deficiencies and significant mortalities at early stages of embryonic development in zebrafish (Danio rerio)
- Authors
- Yilmaz, O., Com, E., Pineau, C., Bobe, J.
- Source
- Full text @ Sci. Rep.
|
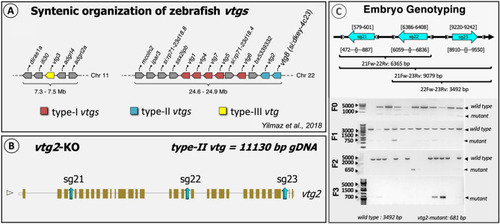
Schematic representation of the general strategy for CRISPR target design. ( |
|
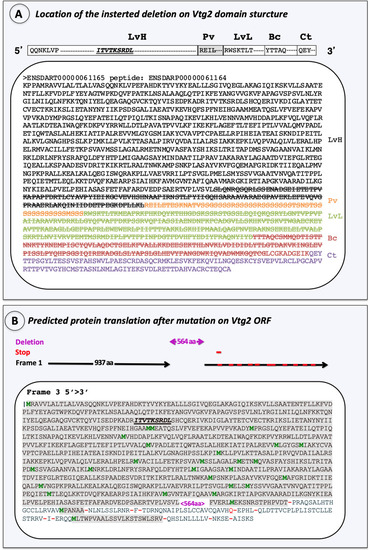
Characterization of the introduced mutation. ( |
|
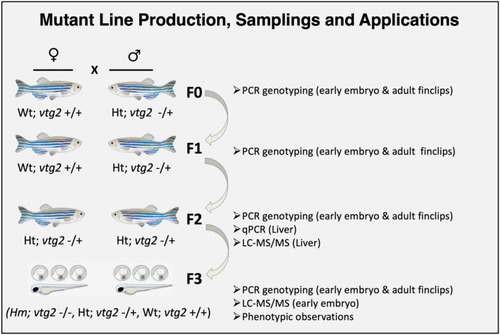
Production of F3 generation |
|
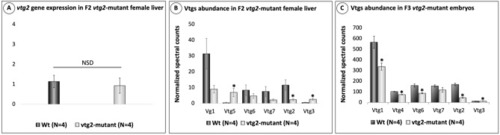
Relative quantification of |
|
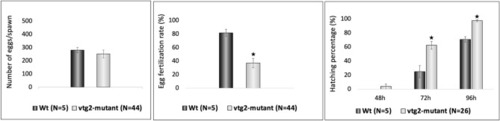
Phenotypic measurements of F2 PHENOTYPE:
|
|
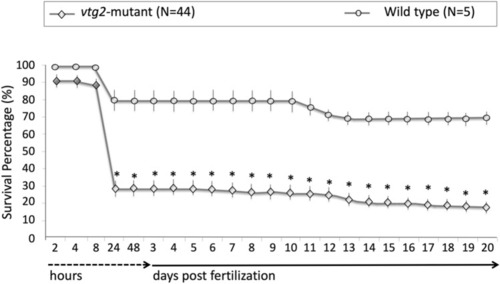
Comparisons of survival percentages for F3 PHENOTYPE:
|
|
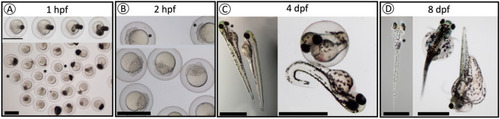
Observed phenotypes of F3 |
|
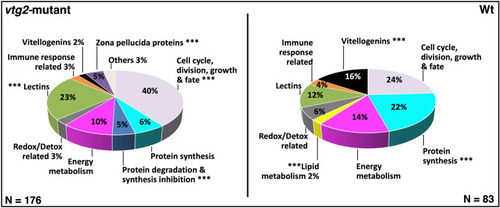
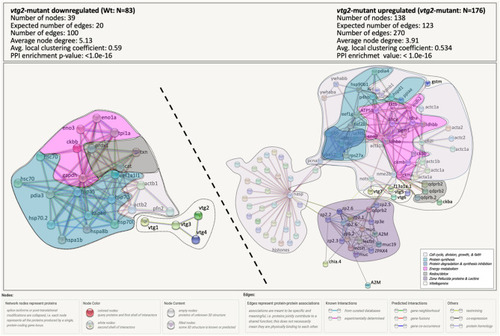
Percent distribution of DEPs. Distribution of differentially regulated proteins among functional categories. |
|
STRING Network Analysis of the differentially regulated proteins in |