- Title
-
Decoding pancreatic endocrine cell differentiation and β cell regeneration in zebrafish
- Authors
- Mi, J., Liu, K.C., Andersson, O.
- Source
- Full text @ Sci Adv
|
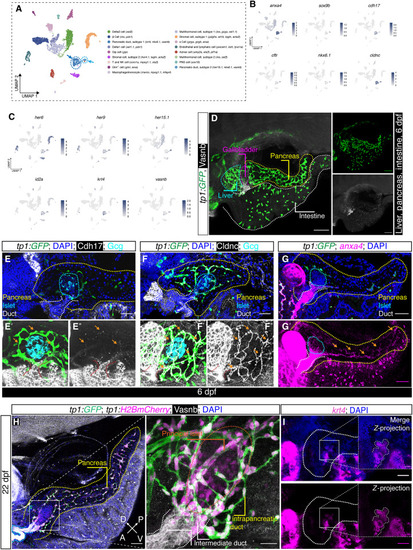
Ductal heterogeneity in the adult zebrafish pancreas. ( |
|
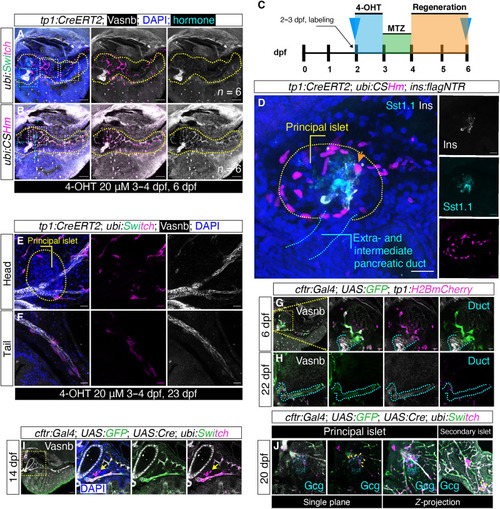
Lineage tracing showed limited Notch-responsive duct–to–endocrine cells conversion at the principal islet. ( |
|
Lineage tracing and targeted cell ablation using ( |
|
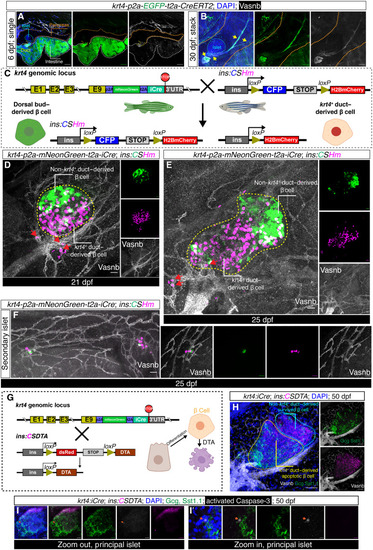
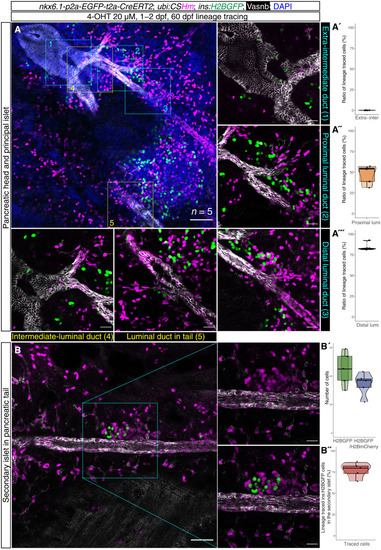
Spatiotemporal-controlled lineage tracing of ( |
|
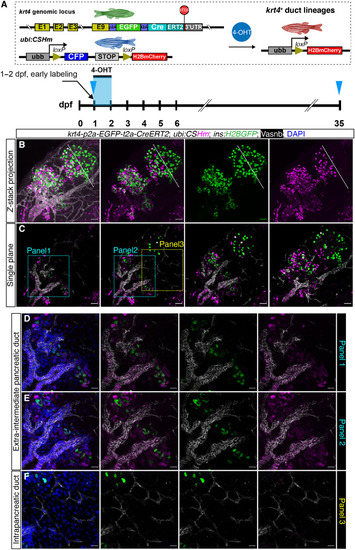
Spatiotemporal-controlled lineage tracing of ( |
|
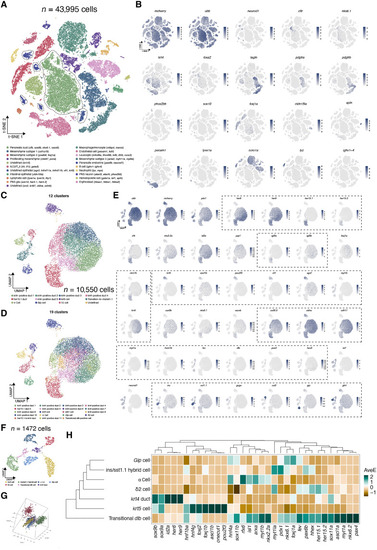
Single-cell transcriptomics highlight distinct molecular signatures in various cell types. ( |
|
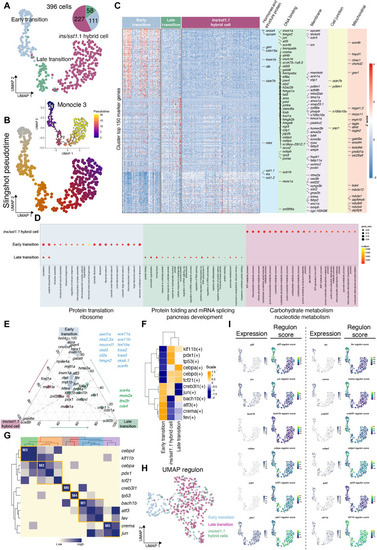
In silico analyses of transition-to- ( |
|
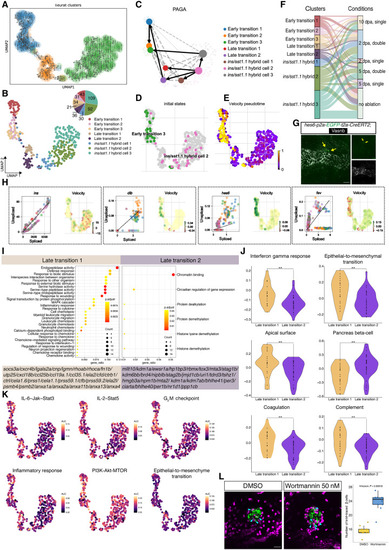
Velocity-based analyses and pathway validation. ( |
|
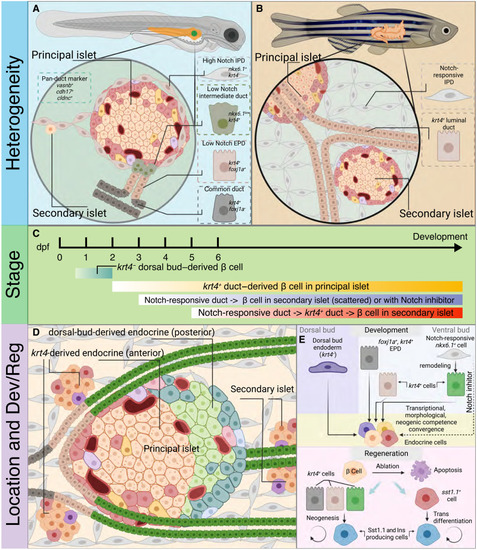
Graphical summary describes the landscape of endocrinogenesis in zebrafish pancreas. ( |