- Title
-
Site and Mechanism of ML252 Inhibition of Kv7 Voltage-Gated Potassium Channels
- Authors
- Kanyo, R., Lamothe, S.M., Urrutia, A., Goodchild, S.J., Allison, W.T., Dean, R., Kurata, H.T.
- Source
- Full text @ Function (Oxf)
|
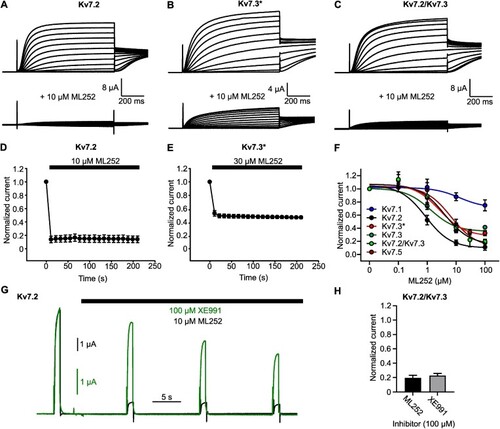
ML252 inhibits Kv7.2 and Kv7.3 homomeric channels with rapid onset. (A to C) Exemplar records from |
|
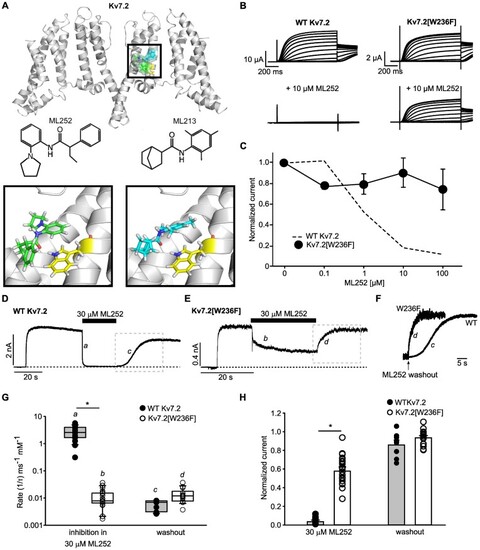
Kv7.2 Trp236 strongly influences ML252 inhibition. (A) Docked poses of ML252 (green) in the cryo-EM structure of Kv7.2 (PDB 7VNP), along with the experimentally reported binding mode of ML213 (cyan). The binding pocket is enlarged in the inset, illustrating the orientation of ML252 (left) or ML213 (right) relative to Trp236 (yellow). (B) Exemplar traces from |
|
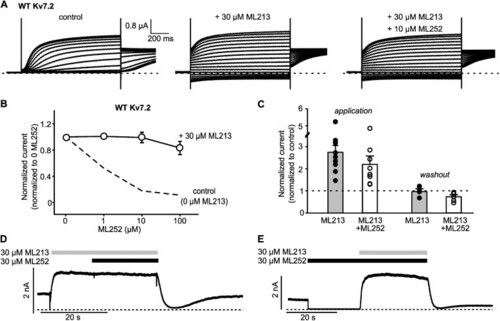
The pore-targeted activator ML213 weakens ML252 inhibition of Kv7.2. (A) Exemplar records of Kv7.2 expressed in |
|
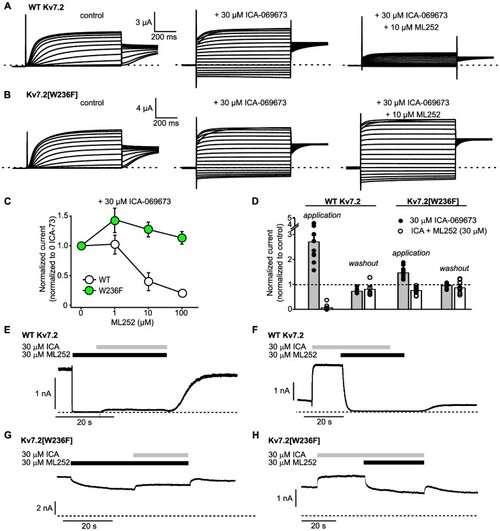
The VSD-targeted activator ICA-069673 does not weaken ML252 inhibition. (A and B) Exemplar records of WT Kv7.2 or Kv7.2[W236F] homomers expressed in |
|
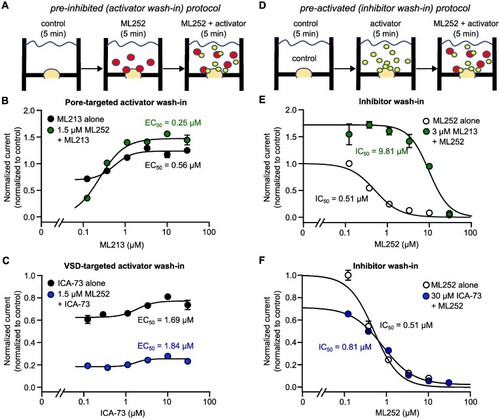
Automated patch clamp analysis of ML252 interactions with Kv7 activators in Kv7.2/Kv7.3 heteromeric channels. (A and D) Drug perfusion paradigms to assess functional outcomes of interactions between ML252 and either ML213 or ICA-069673 (ICA-73). In (A), currents were first recorded in control (no drugs added), following a 5-min incubation in ML252 (at ∼IC50 as determined in |
|
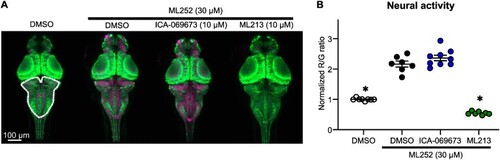
ML252 modifies neuronal activity in zebrafish larvae, and is selectively weakened by the pore-targeted activator ML213. (A) Exemplar images of zebrafish larvae (dorsal view) with neuronal-specific expression of CaMPARI. PC (leading to a higher magenta signal) indicates higher neuronal activity, whereas the absence of PC (weaker magenta signal) indicates low neuronal activity. (B) Summary of PC (red/green ratio) observed in zebrafish larvae treated with ML252 alone or in combination with either ML213 or ICA-069673. Intensities of red (R) and green (G) fluorescence emissions are displayed as red to green ratio and normalized to DMSO controls (quantified in the hindbrain area, dashed white line in panel A) ( |
|
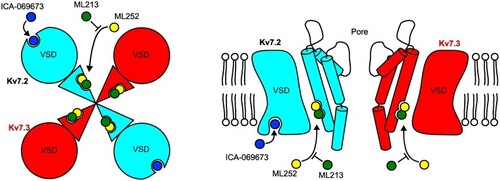
Summary of ML252 inhibition in the context of Kv7 activator binding sites. Mutagenesis and docking suggest that ML252 binds to a similar site as pore-targeted activators including retigabine and ML213 in both Kv7.2 (cyan) and Kv7.3 (red). This underlies less potent ML252-mediated inhibition when applied together with a pore-targeted activator. VSD-targeted activators ztz-240 and ICA-069673 bind to a distinct site (only present in Kv7.2), and therefore do not prevent ML252 inhibition. |