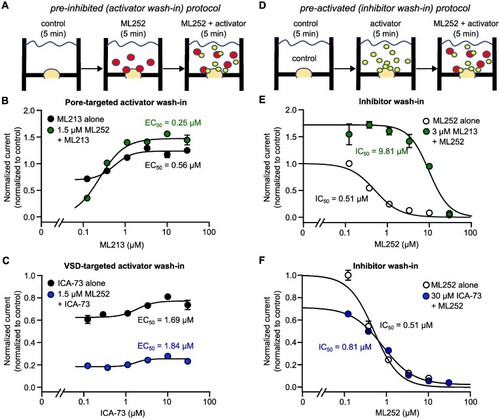
Figure 5.
- ID
- ZDB-FIG-230622-28
- Publication
- Kanyo et al., 2023 - Site and Mechanism of ML252 Inhibition of Kv7 Voltage-Gated Potassium Channels
- Other Figures
- All Figure Page
- Back to All Figure Page
|
Automated patch clamp analysis of ML252 interactions with Kv7 activators in Kv7.2/Kv7.3 heteromeric channels. (A and D) Drug perfusion paradigms to assess functional outcomes of interactions between ML252 and either ML213 or ICA-069673 (ICA-73). In (A), currents were first recorded in control (no drugs added), following a 5-min incubation in ML252 (at ∼IC50 as determined in |